当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational and charge changes induced by l-Arginine and l-lysine increase the solubility of chicken myosin
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2019-04-01 , DOI: 10.1016/j.foodhyd.2018.10.059 Shiyi Li , Linxian Li , Xiaoxu Zhu , Cheng Ning , Kezhou Cai , Cunliu Zhou
Food Hydrocolloids ( IF 11.0 ) Pub Date : 2019-04-01 , DOI: 10.1016/j.foodhyd.2018.10.059 Shiyi Li , Linxian Li , Xiaoxu Zhu , Cheng Ning , Kezhou Cai , Cunliu Zhou
|
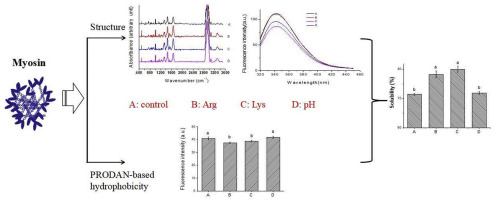
Abstract The paper investigated the mechanism that l -arginine (Arg)/ l -lysine (Lys) affected myosin solubility by spectroscopic technologies and chemical probes. The results showed that Arg and Lys increased myosin solubility. However, neither Arg, nor Lys changed the Raman pattern of myosin. Raman spectra disclosed that Arg and Lys decreased the intensity at 753 cm−1, but increased the intensity at 1,234, 1,448, and 2934 cm−1 and the I853/830 and I3287/3443 ratio, and caused a red-shift at 1656 and 2934 cm−1. Fluorescence spectra showed that Arg/Lys caused the reduced intensity and red-shift peak position of myosin. Arg and Lys slightly affected total reactive sulfhydryl content, increased the ANS-based hydrophobicity and reactive sulfhydryl content, but decreased the PRODAN-based hydrophobicity. Our results collectively demonstrated that Arg and Lys induced myosin to partially unfold and gain more negative charges, which overall enhanced the interaction between myosin and water, ultimately contributing to the increased solubility of myosin.
中文翻译:

l-精氨酸和l-赖氨酸诱导的构象和电荷变化增加了鸡肌球蛋白的溶解度
摘要 本文通过光谱技术和化学探针研究了l-精氨酸(Arg)/l-赖氨酸(Lys)影响肌球蛋白溶解度的机制。结果表明Arg和Lys增加了肌球蛋白溶解度。然而,Arg 和 Lys 都没有改变肌球蛋白的拉曼模式。拉曼光谱显示 Arg 和 Lys 在 753 cm-1 处的强度降低,但在 1,234、1,448 和 2934 cm-1 处的强度以及 I853/830 和 I3287/3443 比值增加,并导致 1656 和2934 厘米-1。荧光光谱显示,Arg/Lys 导致肌球蛋白强度降低和峰位置红移。Arg和Lys对总反应性巯基含量有轻微影响,增加了基于ANS的疏水性和反应性巯基含量,但降低了基于PRODAN的疏水性。
更新日期:2019-04-01
中文翻译:

l-精氨酸和l-赖氨酸诱导的构象和电荷变化增加了鸡肌球蛋白的溶解度
摘要 本文通过光谱技术和化学探针研究了l-精氨酸(Arg)/l-赖氨酸(Lys)影响肌球蛋白溶解度的机制。结果表明Arg和Lys增加了肌球蛋白溶解度。然而,Arg 和 Lys 都没有改变肌球蛋白的拉曼模式。拉曼光谱显示 Arg 和 Lys 在 753 cm-1 处的强度降低,但在 1,234、1,448 和 2934 cm-1 处的强度以及 I853/830 和 I3287/3443 比值增加,并导致 1656 和2934 厘米-1。荧光光谱显示,Arg/Lys 导致肌球蛋白强度降低和峰位置红移。Arg和Lys对总反应性巯基含量有轻微影响,增加了基于ANS的疏水性和反应性巯基含量,但降低了基于PRODAN的疏水性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号