当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Role of Bridging Water and Hydrogen Bonding as Key Determinants of Noncovalent Protein–Carbohydrate Recognition
ChemMedChem ( IF 3.4 ) Pub Date : 2018-11-21 , DOI: 10.1002/cmdc.201800437 Anatoly M. Ruvinsky 1 , Ishita Aloni 2 , Daniel Cappel 3 , Chris Higgs 4 , Kyle Marshall 5 , Piotr Rotkiewicz 1 , Matt Repasky 5 , Victoria A. Feher 4 , Eric Feyfant 1 , Gerhard Hessler 6 , Hans Matter 6
ChemMedChem ( IF 3.4 ) Pub Date : 2018-11-21 , DOI: 10.1002/cmdc.201800437 Anatoly M. Ruvinsky 1 , Ishita Aloni 2 , Daniel Cappel 3 , Chris Higgs 4 , Kyle Marshall 5 , Piotr Rotkiewicz 1 , Matt Repasky 5 , Victoria A. Feher 4 , Eric Feyfant 1 , Gerhard Hessler 6 , Hans Matter 6
Affiliation
|
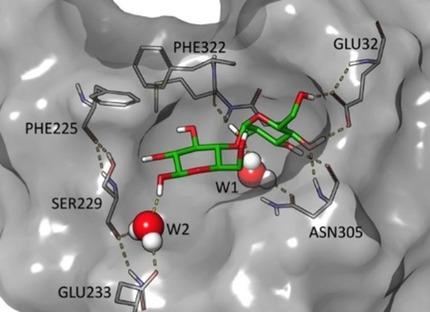
Mechanisms of protein–carbohydrate recognition attract a lot of interest due to their roles in various cellular processes and metabolism disorders. We have performed a large‐scale analysis of protein structures solved in complex with glucose, galactose and their substituted analogues. We found that, on average, sugar molecules establish five hydrogen bonds (HBs) in the binding site, including one to three HBs with bridging water molecules. The free energy contribution of bridging and direct HBs was estimated using the free energy perturbation (FEP+) methodology for mono‐ and disaccharides that bind to l‐ABP, ttGBP, TrmB, hGalectin‐1 and hGalectin‐3. We show that removing hydroxy groups that are engaged in direct HBs with the charged groups of Asp, Arg and Glu residues, protein backbone amide or buried water dramatically decreases binding affinity. In contrast, all solvent‐exposed hydroxy groups and hydroxy groups engaged in HBs with the solvent‐exposed bridging water molecules contribute weakly to binding affinity and so can be replaced to optimize ligand potency. Finally, we rationalize an effect of binding site water replacement on the binding affinity to l‐ABP.
中文翻译:

桥接水和氢键作为非共价蛋白-碳水化合物识别的关键决定因素
蛋白质-碳水化合物识别的机制因其在各种细胞过程和代谢紊乱中的作用而引起了人们的极大兴趣。我们对与葡萄糖,半乳糖及其取代类似物复合的蛋白质结构进行了大规模分析。我们发现,平均而言,糖分子在结合位点建立五个氢键(HBs),包括一到三个带有桥连水分子的HBs。对于结合至l的单糖和二糖,使用自由能扰动(FEP +)方法估算了桥接和直接HBs的自由能贡献-ABP,ttGBP,TrmB,hGalectin-1和hGalectin-3。我们显示,除去与直接的HBs结合的带有Asp,Arg和Glu残基,蛋白质主链酰胺或掩埋水的带电基团的羟基会大大降低结合亲和力。相反,所有暴露于溶剂的羟基和与暴露于溶剂的桥联水分子结合在HBs中的羟基对结合亲和力的贡献都很弱,因此可以进行取代以优化配体效价。最后,我们合理化了结合位点水置换对l- ABP结合亲和力的影响。
更新日期:2018-11-21
中文翻译:

桥接水和氢键作为非共价蛋白-碳水化合物识别的关键决定因素
蛋白质-碳水化合物识别的机制因其在各种细胞过程和代谢紊乱中的作用而引起了人们的极大兴趣。我们对与葡萄糖,半乳糖及其取代类似物复合的蛋白质结构进行了大规模分析。我们发现,平均而言,糖分子在结合位点建立五个氢键(HBs),包括一到三个带有桥连水分子的HBs。对于结合至l的单糖和二糖,使用自由能扰动(FEP +)方法估算了桥接和直接HBs的自由能贡献-ABP,ttGBP,TrmB,hGalectin-1和hGalectin-3。我们显示,除去与直接的HBs结合的带有Asp,Arg和Glu残基,蛋白质主链酰胺或掩埋水的带电基团的羟基会大大降低结合亲和力。相反,所有暴露于溶剂的羟基和与暴露于溶剂的桥联水分子结合在HBs中的羟基对结合亲和力的贡献都很弱,因此可以进行取代以优化配体效价。最后,我们合理化了结合位点水置换对l- ABP结合亲和力的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号