Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-10-31 , DOI: 10.1016/j.cplett.2018.10.073 Hongning Zheng , Han Liu , Jinyuan Hu , Fei Xu
|
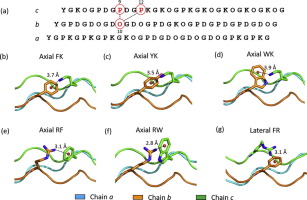
As one of non-covalent forces to stabilize protein, cation-π interactions in collagen have been received much attention. Three chains of collagen form its characteristic secondary structure, triple helices. Based on a collagen heterotrimer, abc, a library containing 15 mutant peptides was built to characterize the stabilizing effects of 24 pairwise cation-π interactions. The six stabilizing pairs, axial FK, YK, WK, RF, and RW, and lateral RF, have been identified, among which only axial RF frequently occurs in natural collagen sequences. This suggests that cation-π interactions might not be optimized in natural collagen.
中文翻译:

使用胶原异三聚体筛选阳离子-π相互作用以稳定三重螺旋。
作为稳定蛋白质的非共价作用力之一,胶原蛋白中的阳离子-π相互作用已引起广泛关注。胶原蛋白的三条链形成其特征性的二级结构,三重螺旋。基于胶原异源三聚体abc,构建了包含15个突变体肽的文库,以表征24对阳离子-π相互作用的稳定作用。已经确定了六个稳定对,即轴向FK,YK,WK,RF和RW,以及横向RF,其中在天然胶原序列中仅经常出现轴向RF。这表明在天然胶原蛋白中可能无法优化阳离子-π相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号