Catalysis Communications ( IF 3.4 ) Pub Date : 2018-10-31 , DOI: 10.1016/j.catcom.2018.10.022 Guang Yang , Ran Cang , Li-Qun Shen , Feng Xue , He Huang , Zhi-Gang Zhang
|
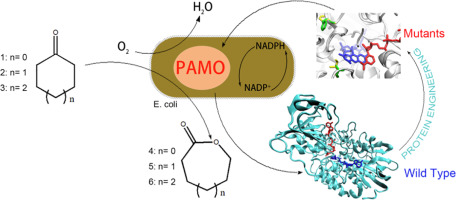
Baeyer-Villiger monooxygenases (BVMOs) convert ketones into lactones that have important applications in polymer synthesis. Here we report on expanding the substrate scope of a thermostable phenylacetone monooxygenase (PAMO) to cyclohexanone by using site-directed mutagenesis. Several new mutants were found to be active with cyclohexanone for which wild-type PAMO shows no activity. The best mutants could convert 10 mM cyclohexanone completely within 12 h, and their catalytic properties were characterized subsequently. In addition to cyclohexanone, several other cyclic ketones were also identified as substrate for the new evolved mutants. These results expand the biocatalytic toolbox for further feasible applications.
中文翻译:

通过蛋白质工程扩大苯丙氨酸单双氧合酶从Therobifida fusca向环己酮的底物范围
Baeyer-Villiger单加氧酶(BVMOs)将酮转化为内酯,这在聚合物合成中具有重要的应用。在这里我们报告通过使用定点诱变将热稳定的苯丙酮单加氧酶(PAMO)的底物范围扩展到环己酮。发现一些新的突变体对环己酮具有活性,而野生型PAMO对环己酮没有活性。最好的突变体可在12 h内完全转化10 mM环己酮,并随后表征其催化性能。除环己酮外,其他几种环酮也被确定为新进化突变体的底物。这些结果扩大了生物催化工具箱的应用范围。











































 京公网安备 11010802027423号
京公网安备 11010802027423号