当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A two-step telescoped continuous flow switchable process leading to nitriles, diaziridine or hydrazine derivatives†
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-11-01 00:00:00 , DOI: 10.1039/c8re00129d Audun Drageset 1, 2, 3, 4 , Nils Åge Frøystein 1, 2, 3, 4 , Karl Wilhelm Törnroos 1, 2, 3, 4 , Hans-René Bjørsvik 1, 2, 3, 4
Reaction Chemistry & Engineering ( IF 3.4 ) Pub Date : 2018-11-01 00:00:00 , DOI: 10.1039/c8re00129d Audun Drageset 1, 2, 3, 4 , Nils Åge Frøystein 1, 2, 3, 4 , Karl Wilhelm Törnroos 1, 2, 3, 4 , Hans-René Bjørsvik 1, 2, 3, 4
Affiliation
|
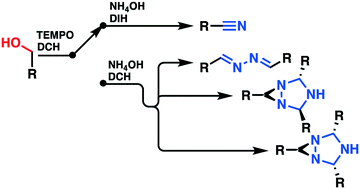
Primary and benzylic alcohols were cost-effectively transformed into their corresponding nitriles using a classical batch approach and a continuous flow process implemented on a multi-jet oscillating disk (MJOD) reactor platform. The alcohols as substrates were treated with a (2,2,6,6-tetra-methylpiperidin-1-yl)oxidanyl (TEMPO) free radical as the pre-catalyst with 1,3-dichloro-5,5-dimethylhydantoin (DCH) as the terminal oxidant to produce their corresponding carbonyl compounds. The reaction was conducted at a reaction temperature of 35 °C and a flow reactor residence time of 5 min. This alcohol to carbonyl oxidation step was telescoped with a subsequent step that involved treatment with aqueous ammonium hydroxide and 1,3-diiodo-5,5-dimethylhydantoin (DIH) at a reaction temperature of 65 °C with concomitant oxidation to nitrile using a flow reactor residence time of 15 min. A solvent exchange process was conducted in-between the two synthetic reaction steps by means of an in-line extraction process with ethyl acetate, a step that was concatenated by using an in-line liquid–liquid separation process using a hold-up tank. The second synthetic step was revealed to be tuneable, since four distinct products might be produced at varying degrees of selectivity. When DIH was used as the terminal oxidant, a high selectivity towards the original target nitrile was achieved, but if DIH was replaced with DCH as the terminal oxidant, 1,2-di((E)-benzylidene)hydrazine and two different stereoisomers of the 1,3,5-triazabicyclo[3.1.0]hexane scaffold were produced. The selectivity towards the various products was highly influenced by the reaction temperature. A scope and limitation study of the nitrile process with an assortment of primary alcohols as substrates provided excellent yields which revealed good functional group tolerance.
中文翻译:

两步伸缩式连续流可切换过程,可产生腈,二氮丙啶或肼衍生物†
使用经典间歇方法和在多喷嘴振荡盘(MJOD)反应器平台上实施的连续流工艺,可以经济高效地将伯醇和苯甲醇转化为相应的腈。用(2,2,6,6-四甲基哌啶-1-基)氧烷基(TEMPO)自由基作为前催化剂,用1,3-二氯-5,5-二甲基乙内酰脲(DCH)处理醇作为底物)作为末端氧化剂生产相应的羰基化合物。反应在35℃的反应温度和5分钟的流动反应器停留时间下进行。将该醇转化为羰基的氧化步骤进行下一步,该步骤涉及用氢氧化铵水溶液和1,3-二碘-5的处理,5-二甲基乙内酰脲(DIH)在65°C的反应温度下,伴随15分钟的流动反应器停留时间被氧化为腈。溶剂交换过程是在两个合成反应步骤之间,通过使用乙酸乙酯的在线萃取过程进行的,该步骤通过使用保留罐的在线液-液分离过程进行串联。由于可能以不同程度的选择性产生四种不同的产物,因此第二个合成步骤显示出可调谐性。当将DIH用作末端氧化剂时,可实现对原始目标腈的高选择性,但如果用DCH代替DIH作为末端氧化剂,则1,2-di((溶剂交换过程是在两个合成反应步骤之间,通过使用乙酸乙酯的在线萃取过程进行的,该步骤通过使用保留罐的在线液-液分离过程进行串联。由于可能以不同程度的选择性产生四种不同的产物,因此第二个合成步骤显示出可调谐性。当将DIH用作末端氧化剂时,可实现对原始目标腈的高选择性,但如果用DCH代替DIH作为末端氧化剂,则1,2-di((溶剂交换过程是在两个合成反应步骤之间,通过使用乙酸乙酯的在线萃取过程进行的,该步骤通过使用保留罐的在线液-液分离过程进行串联。由于可能以不同程度的选择性产生四种不同的产物,因此第二个合成步骤显示出可调谐性。当将DIH用作末端氧化剂时,可实现对原始目标腈的高选择性,但如果用DCH代替DIH作为末端氧化剂,则1,2-di((制备了E)-亚苄基)肼和1,3,5-三氮杂双环[3.1.0]己烷骨架的两种不同的立体异构体。对各种产物的选择性受反应温度的影响很大。以各种伯醇为底物的腈过程的范围和限制研究提供了优异的收率,表明具有良好的官能团耐受性。
更新日期:2018-11-01
中文翻译:

两步伸缩式连续流可切换过程,可产生腈,二氮丙啶或肼衍生物†
使用经典间歇方法和在多喷嘴振荡盘(MJOD)反应器平台上实施的连续流工艺,可以经济高效地将伯醇和苯甲醇转化为相应的腈。用(2,2,6,6-四甲基哌啶-1-基)氧烷基(TEMPO)自由基作为前催化剂,用1,3-二氯-5,5-二甲基乙内酰脲(DCH)处理醇作为底物)作为末端氧化剂生产相应的羰基化合物。反应在35℃的反应温度和5分钟的流动反应器停留时间下进行。将该醇转化为羰基的氧化步骤进行下一步,该步骤涉及用氢氧化铵水溶液和1,3-二碘-5的处理,5-二甲基乙内酰脲(DIH)在65°C的反应温度下,伴随15分钟的流动反应器停留时间被氧化为腈。溶剂交换过程是在两个合成反应步骤之间,通过使用乙酸乙酯的在线萃取过程进行的,该步骤通过使用保留罐的在线液-液分离过程进行串联。由于可能以不同程度的选择性产生四种不同的产物,因此第二个合成步骤显示出可调谐性。当将DIH用作末端氧化剂时,可实现对原始目标腈的高选择性,但如果用DCH代替DIH作为末端氧化剂,则1,2-di((溶剂交换过程是在两个合成反应步骤之间,通过使用乙酸乙酯的在线萃取过程进行的,该步骤通过使用保留罐的在线液-液分离过程进行串联。由于可能以不同程度的选择性产生四种不同的产物,因此第二个合成步骤显示出可调谐性。当将DIH用作末端氧化剂时,可实现对原始目标腈的高选择性,但如果用DCH代替DIH作为末端氧化剂,则1,2-di((溶剂交换过程是在两个合成反应步骤之间,通过使用乙酸乙酯的在线萃取过程进行的,该步骤通过使用保留罐的在线液-液分离过程进行串联。由于可能以不同程度的选择性产生四种不同的产物,因此第二个合成步骤显示出可调谐性。当将DIH用作末端氧化剂时,可实现对原始目标腈的高选择性,但如果用DCH代替DIH作为末端氧化剂,则1,2-di((制备了E)-亚苄基)肼和1,3,5-三氮杂双环[3.1.0]己烷骨架的两种不同的立体异构体。对各种产物的选择性受反应温度的影响很大。以各种伯醇为底物的腈过程的范围和限制研究提供了优异的收率,表明具有良好的官能团耐受性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号