当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction mechanism of NO with hydrolysates of NAMI-A: an MD simulation by combining the QM/MM(ABEEM) with the MD-FEP method
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-10-30 , DOI: 10.1002/jcc.25734 Hui Li 1, 2 , Di Wang 1 , Xin Zhao 1 , Li-Nan Lu 1 , Cui Liu 1 , Li-Dong Gong 1 , Dong-Xia Zhao 1 , Zhong-Zhi Yang 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-10-30 , DOI: 10.1002/jcc.25734 Hui Li 1, 2 , Di Wang 1 , Xin Zhao 1 , Li-Nan Lu 1 , Cui Liu 1 , Li-Dong Gong 1 , Dong-Xia Zhao 1 , Zhong-Zhi Yang 1
Affiliation
|
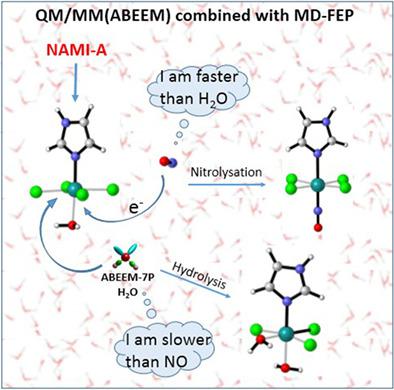
Nitrosylation reaction mechanisms of the hydrolysates of NAMI‐A and hydrolysis reactions of ruthenium nitrosyl complexes were investigated in the triplet state and the singlet state. Activation free energies were calculated by combining the QM/MM(ABEEM) method with free energy perturbation theory, and the explicit solvent environment was simulated by an ABEEMσπ polarizable force field. Our results demonstrate that nitrosylation reactions of the hydrolysates of NAMI‐A occur in both the triplet and the singlet states. The Ru‐N‐O angle of the triplet ruthenium nitrosyl complexes is in the range of 132.0°–138.2°. However, all the ruthenium nitrosyl complexes at the singlet state show an almost linear Ru‐N‐O angle. The nitrosylation reaction happens prior to the hydrolysis reaction for the first‐step hydrolysates. The activation free energies of the nitrosylation reactions show that the H2O‐NO exchange reaction of [RuCl4(Im)(H2O)] in the singlet spin sate is the most likely one. Comparing with the activation free energies of the hydrolysis reactions of the ruthenium nitrosyl complexes, the results indicate that the rate of the DMSO–H2O exchange reaction of [RuCl3(NO)(Im)(DMSO)] is faster than that of [RuCl3(H2O)(Im)(DMSO)] in both the triplet spin state and the singlet spin state. © 2018 Wiley Periodicals, Inc.
中文翻译:

NO与NAMI-A水解物的反应机理:结合QM/MM(ABEEM)和MD-FEP方法的MD模拟
在三重态和单重态下研究了 NAMI-A 水解产物的亚硝基化反应机理和亚硝酰基钌配合物的水解反应。结合QM/MM(ABEEM)方法和自由能微扰理论计算活化自由能,并通过ABEEMσπ极化力场模拟显式溶剂环境。我们的结果表明,NAMI-A 水解产物的亚硝基化反应同时发生在三重态和单重态。三线态亚硝酰基钌配合物的 Ru-N-O 角在 132.0°–138.2° 的范围内。然而,所有处于单线态的亚硝酰基钌配合物都显示出几乎线性的 Ru-N-O 角。亚硝基化反应发生在第一步水解物的水解反应之前。亚硝基化反应的活化自由能表明,单线态自旋态中 [RuCl4(Im)(H2O)] 的 H2O-NO 交换反应是最有可能的。与亚硝酰基钌配合物水解反应的活化自由能相比,结果表明[RuCl3(NO)(Im)(DMSO)]的DMSO-H2O交换反应速率比[RuCl3( H2O)(Im)(DMSO)] 处于三重自旋态和单重自旋态。© 2018 Wiley Periodicals, Inc. 结果表明 [RuCl3(NO)(Im)(DMSO)] 的 DMSO-H2O 交换反应的速率比 [RuCl3(H2O)(Im)(DMSO)] 在三重自旋态和单重自旋状态。© 2018 Wiley Periodicals, Inc. 结果表明 [RuCl3(NO)(Im)(DMSO)] 的 DMSO-H2O 交换反应的速率比 [RuCl3(H2O)(Im)(DMSO)] 在三重自旋态和单重自旋状态。© 2018 Wiley Periodicals, Inc.
更新日期:2018-10-30
中文翻译:

NO与NAMI-A水解物的反应机理:结合QM/MM(ABEEM)和MD-FEP方法的MD模拟
在三重态和单重态下研究了 NAMI-A 水解产物的亚硝基化反应机理和亚硝酰基钌配合物的水解反应。结合QM/MM(ABEEM)方法和自由能微扰理论计算活化自由能,并通过ABEEMσπ极化力场模拟显式溶剂环境。我们的结果表明,NAMI-A 水解产物的亚硝基化反应同时发生在三重态和单重态。三线态亚硝酰基钌配合物的 Ru-N-O 角在 132.0°–138.2° 的范围内。然而,所有处于单线态的亚硝酰基钌配合物都显示出几乎线性的 Ru-N-O 角。亚硝基化反应发生在第一步水解物的水解反应之前。亚硝基化反应的活化自由能表明,单线态自旋态中 [RuCl4(Im)(H2O)] 的 H2O-NO 交换反应是最有可能的。与亚硝酰基钌配合物水解反应的活化自由能相比,结果表明[RuCl3(NO)(Im)(DMSO)]的DMSO-H2O交换反应速率比[RuCl3( H2O)(Im)(DMSO)] 处于三重自旋态和单重自旋态。© 2018 Wiley Periodicals, Inc. 结果表明 [RuCl3(NO)(Im)(DMSO)] 的 DMSO-H2O 交换反应的速率比 [RuCl3(H2O)(Im)(DMSO)] 在三重自旋态和单重自旋状态。© 2018 Wiley Periodicals, Inc. 结果表明 [RuCl3(NO)(Im)(DMSO)] 的 DMSO-H2O 交换反应的速率比 [RuCl3(H2O)(Im)(DMSO)] 在三重自旋态和单重自旋状态。© 2018 Wiley Periodicals, Inc.









































 京公网安备 11010802027423号
京公网安备 11010802027423号