Nature Energy ( IF 49.7 ) Pub Date : 2018-10-29 , DOI: 10.1038/s41560-018-0268-z Wenbo Gao , Jianping Guo , Peikun Wang , Qianru Wang , Fei Chang , Qijun Pei , Weijin Zhang , Lin Liu , Ping Chen
|
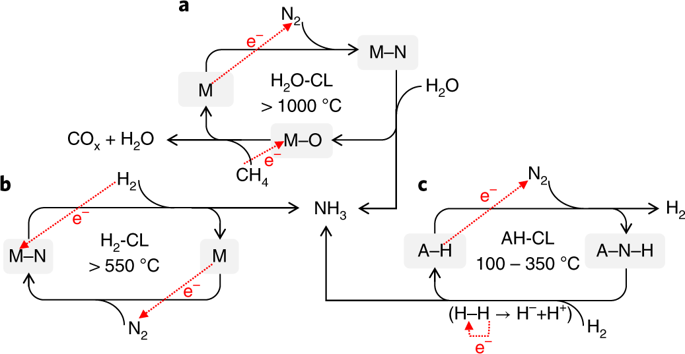
Ammonia is a promising carbon-free energy carrier, but is currently synthesized industrially under harsh conditions. Synthesizing ammonia using lower temperatures and pressures could therefore improve its prospects as a chemical means to store and transport energy. Here we report that alkali and alkaline earth metal imides function as nitrogen carriers that mediate ammonia production via a two-step chemical looping process operating under mild conditions. Nitrogen is first fixed through the reduction of N2 by alkali or alkaline earth metal hydrides to form imides and, subsequently, the imides are hydrogenated to produce NH3 and regenerate the metal hydrides. The oxidation state of hydrogen therefore switches between −1 (hydride), 0 (H2) and +1 (imide and NH3). Late 3d metals accelerate the reaction rates of both steps. The chemical loop mediated by BaNH and catalysed by Ni produces NH3 at 100 °C and atmospheric pressure.
中文翻译:

通过基于金属酰亚胺作为氮载体的化学成环工艺生产氨
氨是一种有前途的无碳能源载体,但目前在苛刻的条件下工业合成。因此,在较低的温度和压力下合成氨可以改善其作为存储和运输能量的化学手段的前景。在这里,我们报告说碱金属和碱土金属的酰亚胺充当氮载体,通过在温和条件下运行的两步化学循环过程来介导氨的产生。氮首先通过碱金属或碱土金属氢化物还原N 2来固定,以形成酰亚胺,然后将酰亚胺氢化以生成NH 3并使金属氢化物再生。氢的氧化态因此在-1(氢化物),0(H 2)和+1(酰亚胺和NH )之间切换3)。晚期3d金属会加快两个步骤的反应速度。由BaNH介导并由Ni催化的化学环在100°C和大气压下产生NH 3。











































 京公网安备 11010802027423号
京公网安备 11010802027423号