当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Deprotonative α-Formylation of Heteroarenes by an Amide Base Generated in Situ from Tetramethylammonium Fluoride and Tris(trimethylsilyl)amine
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-10-29 00:00:00 , DOI: 10.1021/acs.oprd.8b00247 Masanori Shigeno 1 , Yuki Fujii 1 , Akihisa Kajima 1 , Kanako Nozawa-Kumada 1 , Yoshinori Kondo 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-10-29 00:00:00 , DOI: 10.1021/acs.oprd.8b00247 Masanori Shigeno 1 , Yuki Fujii 1 , Akihisa Kajima 1 , Kanako Nozawa-Kumada 1 , Yoshinori Kondo 1
Affiliation
|
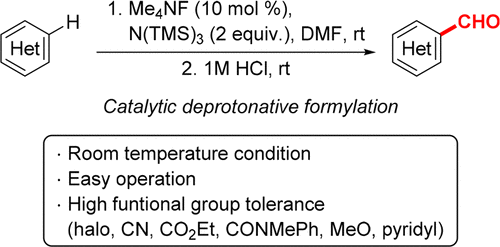
Heteroarene formylations in DMF solution proceed in the presence of an amide base catalyst generated in situ from tetramethylammonium fluoride (TMAF) and tris(trimethylsilyl)amine (N(TMS)3). The reaction proceeds at room temperature and has an operationally simple procedure. Various heteroarenes, including benzothiophene, thiophene, benzothiazole, oxazole, and indole derivatives, can be formylated with high functional group tolerance.
中文翻译:

四甲基氟化铵和三(三甲基甲硅烷基)胺原位生成的酰胺基催化杂芳烃的催化质子化α-甲酰化
DMF溶液中的杂芳烃甲酰化反应是在由四甲基氟化铵(TMAF)和三(三甲基甲硅烷基)胺(N(TMS)3)原位生成的酰胺基催化剂存在下进行的。反应在室温下进行,并且操作简单。各种杂芳烃,包括苯并噻吩,噻吩,苯并噻唑,恶唑和吲哚衍生物,可以被甲酰基化,具有高度的官能团耐受性。
更新日期:2018-10-29
中文翻译:

四甲基氟化铵和三(三甲基甲硅烷基)胺原位生成的酰胺基催化杂芳烃的催化质子化α-甲酰化
DMF溶液中的杂芳烃甲酰化反应是在由四甲基氟化铵(TMAF)和三(三甲基甲硅烷基)胺(N(TMS)3)原位生成的酰胺基催化剂存在下进行的。反应在室温下进行,并且操作简单。各种杂芳烃,包括苯并噻吩,噻吩,苯并噻唑,恶唑和吲哚衍生物,可以被甲酰基化,具有高度的官能团耐受性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号