当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dative and electron-sharing bonding in transition metal compounds
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-10-26 , DOI: 10.1002/jcc.25584 Paul Jerabek 1 , Peter Schwerdtfeger 1 , Gernot Frenking 2, 3
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-10-26 , DOI: 10.1002/jcc.25584 Paul Jerabek 1 , Peter Schwerdtfeger 1 , Gernot Frenking 2, 3
Affiliation
|
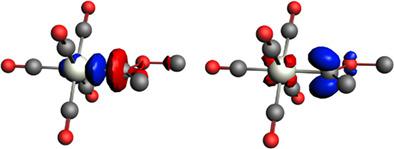
Quantum chemical calculations using density functional theory at the BP86‐D3(BJ)/def2‐TZVPP level of theory are reported for transition metal compounds [TM]‐L in high and low oxidation states involving carbene, carbyne, alkene, and alkyne ligands L. The nature of the [TM]‐L bond is analyzed with the energy decomposition analysis ‐ natural orbitals for chemical valence (EDA‐NOCV) method. The calculations reveal that transition metal compounds with ligands, that are typically classified as donor–acceptor complexes possessing dative bonds (Fischer‐type carbenes and carbynes, alkene, and alkyne complexes) or as TM compounds with electron‐sharing bonds (Schrock‐type carbenes and carbynes, metallacyclopropanes, and metallacyclopropenes), exhibit significant differences between the orbital interactions when closed‐shell or open shell fragments are used. Fischer‐type carbene complexes have much lower orbital interaction (ΔEorb) values when singlet fragments are employed compared to triplet fragments. In contrast, singlet and triplet fragments of Schrock‐type carbene complexes give similar ΔEorb values. The best description for Fischer‐type carbyne complexes is found for neutral fragments in their electronic doublet state, which engage in a mixture of dative bonding (σ donation and π backdonation) and one electron‐sharing π bond. The EDA‐NOCV calculations of Schrock‐type carbynes using open‐shell species in their quartet electronic state give similar ΔEorb values as neutral fragments in their electronic doublet state. Alkene and alkyne complexes, but also metallacyclic species, are best described with singlet fragments, but the difference between the ΔEorb values for dative bonding and electron‐sharing bonding using triplet fragments becomes much smaller for molecules that are considered as metallacycles. © 2018 Wiley Periodicals, Inc.
中文翻译:

过渡金属化合物中的配价和电子共享键
使用密度泛函理论在 BP86-D3(BJ)/def2-TZVPP 理论水平对过渡金属化合物 [TM]-L 进行量子化学计算,涉及卡宾、卡宾、烯烃和炔配体 L . [TM]-L 键的性质通过能量分解分析 - 化学价的自然轨道 (EDA-NOCV) 方法进行分析。计算表明,具有配体的过渡金属化合物,通常被归类为具有配价键的供体-受体配合物(Fischer 型卡宾和卡宾、烯烃和炔配合物)或具有电子共享键的 TM 化合物(Schrock 型卡宾)和卡宾、金属环丙烷和金属环丙烯),当使用闭壳或开壳碎片时,轨道相互作用之间表现出显着差异。与三线态碎片相比,当使用单线态碎片时,Fischer 型卡宾配合物的轨道相互作用 (ΔEorb) 值要低得多。相比之下,Schrock 型卡宾配合物的单线态和三线态片段具有相似的 ΔEorb 值。Fischer 型碳炔配合物的最佳描述是处于双电子态的中性碎片,它们参与配位键(σ 捐赠和 π 回赠)和一个电子共享 π 键的混合物。使用四重电子态的开壳物质对 Schrock 型卡宾的 EDA-NOCV 计算得出与电子双重态的中性碎片相似的 ΔEorb 值。烯烃和炔烃配合物,以及金属环物种,最好用单线态片段来描述,但是对于被认为是金属环的分子,使用三线态片段的配价键合和电子共享键合的 ΔEorb 值之间的差异变得小得多。© 2018 Wiley Periodicals, Inc.
更新日期:2018-10-26
中文翻译:

过渡金属化合物中的配价和电子共享键
使用密度泛函理论在 BP86-D3(BJ)/def2-TZVPP 理论水平对过渡金属化合物 [TM]-L 进行量子化学计算,涉及卡宾、卡宾、烯烃和炔配体 L . [TM]-L 键的性质通过能量分解分析 - 化学价的自然轨道 (EDA-NOCV) 方法进行分析。计算表明,具有配体的过渡金属化合物,通常被归类为具有配价键的供体-受体配合物(Fischer 型卡宾和卡宾、烯烃和炔配合物)或具有电子共享键的 TM 化合物(Schrock 型卡宾)和卡宾、金属环丙烷和金属环丙烯),当使用闭壳或开壳碎片时,轨道相互作用之间表现出显着差异。与三线态碎片相比,当使用单线态碎片时,Fischer 型卡宾配合物的轨道相互作用 (ΔEorb) 值要低得多。相比之下,Schrock 型卡宾配合物的单线态和三线态片段具有相似的 ΔEorb 值。Fischer 型碳炔配合物的最佳描述是处于双电子态的中性碎片,它们参与配位键(σ 捐赠和 π 回赠)和一个电子共享 π 键的混合物。使用四重电子态的开壳物质对 Schrock 型卡宾的 EDA-NOCV 计算得出与电子双重态的中性碎片相似的 ΔEorb 值。烯烃和炔烃配合物,以及金属环物种,最好用单线态片段来描述,但是对于被认为是金属环的分子,使用三线态片段的配价键合和电子共享键合的 ΔEorb 值之间的差异变得小得多。© 2018 Wiley Periodicals, Inc.











































 京公网安备 11010802027423号
京公网安备 11010802027423号