当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial redox processes in memristive devices based on valence change and electrochemical metallization
Faraday Discussions ( IF 3.3 ) Pub Date : 2018-10-24 , DOI: 10.1039/c8fd00113h Keqin Liu 1, 2, 3, 4, 5 , Liang Qin 5, 6, 7, 8, 9 , Xiaoxian Zhang 5, 6, 7, 8, 9 , Jiadi Zhu 1, 2, 3, 4, 5 , Xinhao Sun 1, 2, 3, 4, 5 , Ke Yang 1, 2, 3, 4, 5 , Yimao Cai 1, 2, 3, 4, 5 , Yuchao Yang 1, 2, 3, 4, 5 , Ru Huang 1, 2, 3, 4, 5
Faraday Discussions ( IF 3.3 ) Pub Date : 2018-10-24 , DOI: 10.1039/c8fd00113h Keqin Liu 1, 2, 3, 4, 5 , Liang Qin 5, 6, 7, 8, 9 , Xiaoxian Zhang 5, 6, 7, 8, 9 , Jiadi Zhu 1, 2, 3, 4, 5 , Xinhao Sun 1, 2, 3, 4, 5 , Ke Yang 1, 2, 3, 4, 5 , Yimao Cai 1, 2, 3, 4, 5 , Yuchao Yang 1, 2, 3, 4, 5 , Ru Huang 1, 2, 3, 4, 5
Affiliation
|
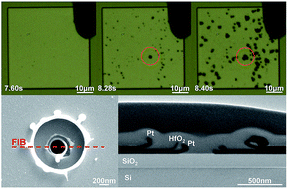
Memristive devices based on electrochemical processes are promising candidates for next-generation memory and neuromorphic applications. The redox processes happening at the interfaces are crucial steps for the ionization as well as generation of counter charges, and are thus indispensable for successful resistive switching, but their detailed mechanism has not been fully clarified. Here, we study the interfacial redox reactions in the forming process of memristive devices based on valence change and electrochemical metallization, using high-resolution electron microscopy and electrostatic force microscopy observations. We show direct evidence for the anodic oxidation of oxygen ions and cathodic reduction of moisture in HfO2- and Ta2O5-based valence change cells, which could take place in different horizontal locations. We further found that the anodic reactions always led to more pronounced structural damage to the electrode, indicating the possibility of additional cathodic reactions without producing gaseous products. When an active electrode is present, oxidation of metal atoms takes place at the anodic interface instead. Further investigations on electrochemical metallization cells have identified Cu ionization and moisture reduction as the anodic and cathodic reactions, respectively, and formation of Cu nuclei at the cathodic interface was directly observed. These findings with microscopic evidence could facilitate future development of memristive devices.
中文翻译:

基于价态变化和电化学金属化的忆阻器件界面氧化还原过程
基于电化学过程的忆阻器件是下一代记忆和神经形态应用的有希望的候选者。发生在界面上的氧化还原过程是电离以及产生反电荷的关键步骤,因此对于成功的电阻切换是必不可少的,但是其详细机理尚未完全阐明。在这里,我们使用高分辨率电子显微镜和静电力显微镜观察,研究了基于价态变化和电化学金属化的忆阻器件形成过程中的界面氧化还原反应。我们显示了HfO 2-和Ta 2 O 5中氧离子的阳极氧化和水分的阴极还原的直接证据。-基于价的变化单元,其可以发生在不同的水平位置。我们进一步发现,阳极反应总是导致对电极的更明显的结构破坏,这表明在不产生气态产物的情况下进行其他阴极反应的可能性。当存在活性电极时,金属原子的氧化反而发生在阳极界面上。对电化学金属化电池的进一步研究已将Cu电离和水分减少分别确定为阳极和阴极反应,并直接观察到在阴极界面处Cu核的形成。这些具有微观证据的发现可以促进忆阻装置的未来发展。
更新日期:2019-02-19
中文翻译:

基于价态变化和电化学金属化的忆阻器件界面氧化还原过程
基于电化学过程的忆阻器件是下一代记忆和神经形态应用的有希望的候选者。发生在界面上的氧化还原过程是电离以及产生反电荷的关键步骤,因此对于成功的电阻切换是必不可少的,但是其详细机理尚未完全阐明。在这里,我们使用高分辨率电子显微镜和静电力显微镜观察,研究了基于价态变化和电化学金属化的忆阻器件形成过程中的界面氧化还原反应。我们显示了HfO 2-和Ta 2 O 5中氧离子的阳极氧化和水分的阴极还原的直接证据。-基于价的变化单元,其可以发生在不同的水平位置。我们进一步发现,阳极反应总是导致对电极的更明显的结构破坏,这表明在不产生气态产物的情况下进行其他阴极反应的可能性。当存在活性电极时,金属原子的氧化反而发生在阳极界面上。对电化学金属化电池的进一步研究已将Cu电离和水分减少分别确定为阳极和阴极反应,并直接观察到在阴极界面处Cu核的形成。这些具有微观证据的发现可以促进忆阻装置的未来发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号