当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Investigation on the Mechanism and Kinetics of Atmospheric Reaction of Methyldichloroacetate with Hydroxyl Radical
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-10-23 00:00:00 , DOI: 10.1021/acs.jpca.8b05223 M. Gnanaprakasam 1 , L. Sandhiya 2 , K. Senthilkumar 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-10-23 00:00:00 , DOI: 10.1021/acs.jpca.8b05223 M. Gnanaprakasam 1 , L. Sandhiya 2 , K. Senthilkumar 1
Affiliation
|
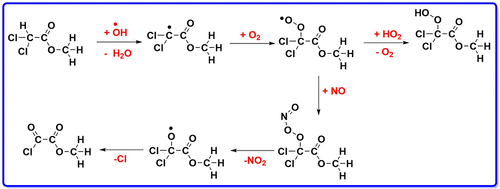
The atmospheric reaction of methyldichloroacetate (MDCA) with OH radical is studied using electronic structure calculations. Five different pathways were considered for the initial reactions, which results in the formation of alkyl radical of MDCA along with H2O, HOCl, and CH3O•. Among the five pathways studied, the α-carbon atom (−CHCl2 site) H atom abstraction reaction, which leads to the formation of the alkyl radical intermediate •CCl2C(O)OCH3 (I1) is found to be more favorable with an energy barrier of 7.3 kcal/mol, and Cl-atom abstraction reaction is having high energy barrier of 21.3 kcal/mol at M06-2X/6-311++G(2df,2p) level. The calculated thermochemical parameters show that except Cl-atom abstraction channel the other initial reaction channels are highly exothermic. The rate constant is calculated for the initial H atom abstraction reactions using canonical variational transition state theory over the temperature range of 278 to 350 K. The Arrhenius plot shows positive temperature dependence for both the reactions. The results from the calculated thermochemical parameters and rate constants show that the formation of the alkyl radical intermediate (I1) is more favorable with the rate constant of 2.07 × 10–13 cm3 molecule–1 s–1 at 298 K. The calculated atmospheric lifetime of MDCA is 28 days at normal atmospheric OH concentration. The results obtained from secondary reactions show that the major product formed from the oxidation chemistry of MDCA is methyl-2-chloro-2-oxoacetate (or) methyl oxalyl chloride.
中文翻译:

二氯乙酸甲酯与羟自由基反应机理及动力学的理论研究
使用电子结构计算研究了二氯乙酸甲酯(MDCA)与OH自由基的大气反应。初始反应考虑了五种不同的途径,导致MDCA的烷基与H 2 O,HOCl和CH 3 O •一起形成。在研究的五种途径中,α-碳原子(-CHCl 2位点)H原子抽象反应导致形成烷基自由基中间体• CCl 2 C(O)OCH 3发现(I1)的势垒为7.3 kcal / mol更有利,Cl原子的抽象反应在M06-2X / 6-311 ++ G(2df,2p)下具有21.3 kcal / mol的高能垒) 等级。所计算的热化学参数表明,除了Cl-原子提取通道外,其他初始反应通道都高度放热。在278至350 K的温度范围内,使用规范变分跃迁状态理论计算了初始H原子抽象反应的速率常数。Arrhenius图显示了这两个反应的正温度依赖性。由计算的热化学参数和速率常数得出的结果表明,烷基自由基中间体(I1)的形成更有利,速率常数为2.07×10 –13 cm 3在298 K时,分子–1 s –1。在正常大气OH浓度下,MDCA的计算大气寿命为28天。从次级反应获得的结果表明,由MDCA的氧化化学反应形成的主要产物是-2-氯-2-氧代乙酸甲酯(或)草酰氯甲基。
更新日期:2018-10-23
中文翻译:

二氯乙酸甲酯与羟自由基反应机理及动力学的理论研究
使用电子结构计算研究了二氯乙酸甲酯(MDCA)与OH自由基的大气反应。初始反应考虑了五种不同的途径,导致MDCA的烷基与H 2 O,HOCl和CH 3 O •一起形成。在研究的五种途径中,α-碳原子(-CHCl 2位点)H原子抽象反应导致形成烷基自由基中间体• CCl 2 C(O)OCH 3发现(I1)的势垒为7.3 kcal / mol更有利,Cl原子的抽象反应在M06-2X / 6-311 ++ G(2df,2p)下具有21.3 kcal / mol的高能垒) 等级。所计算的热化学参数表明,除了Cl-原子提取通道外,其他初始反应通道都高度放热。在278至350 K的温度范围内,使用规范变分跃迁状态理论计算了初始H原子抽象反应的速率常数。Arrhenius图显示了这两个反应的正温度依赖性。由计算的热化学参数和速率常数得出的结果表明,烷基自由基中间体(I1)的形成更有利,速率常数为2.07×10 –13 cm 3在298 K时,分子–1 s –1。在正常大气OH浓度下,MDCA的计算大气寿命为28天。从次级反应获得的结果表明,由MDCA的氧化化学反应形成的主要产物是-2-氯-2-氧代乙酸甲酯(或)草酰氯甲基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号