当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chromium Oxynitride Electrocatalysts for Electrochemical Synthesis of Ammonia Under Ambient Conditions
Small Methods ( IF 12.4 ) Pub Date : 2018-10-21 , DOI: 10.1002/smtd.201800324 Yao Yao 1, 2 , Qi Feng 1 , Shangqian Zhu 2 , Jiadong Li 2 , Yuze Yao 2 , Yajun Wang 3 , Qi Wang 1 , Meng Gu 1 , Haijiang Wang 3 , Hui Li 1 , Xiao‐Zi Yuan 4 , Minhua Shao 2
Small Methods ( IF 12.4 ) Pub Date : 2018-10-21 , DOI: 10.1002/smtd.201800324 Yao Yao 1, 2 , Qi Feng 1 , Shangqian Zhu 2 , Jiadong Li 2 , Yuze Yao 2 , Yajun Wang 3 , Qi Wang 1 , Meng Gu 1 , Haijiang Wang 3 , Hui Li 1 , Xiao‐Zi Yuan 4 , Minhua Shao 2
Affiliation
|
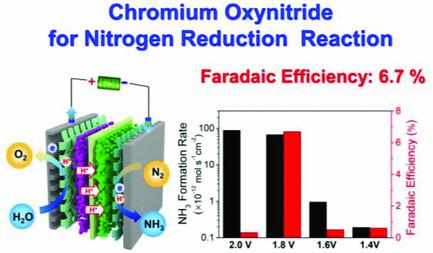
The electrochemical synthesis of ammonia via nitrogen reduction reaction (NRR) has received much attention as a more environmentally friendly and less energy consuming technology than the conventional Haber–Bosch process. The catalytic activities of all NRR electrocatalysts reported so far, however, are very low under ambient conditions. In this study, partially oxidized chromium nitride (chromium oxynitride) nanoparticles are synthesized and their NRR activities are evaluated in a proton exchange membrane electrolyzer under ambient conditions. The highest ammonia formation rate of 8.9 × 10−11 mol s−1 cm−2 and 15.56 µg h−1 mg−1cat are achieved at a cell voltage of 2.0 V. The highest Faradaic efficiency of 6.7% is achieved at a cell voltage of 1.8 V. The findings demonstrate that metal nitride–based materials can be promising electrocatalysts toward NRR and could guide rational design of more advanced catalysts for various reactions.
中文翻译:

常温条件下用于电化学合成氨的氧氮化铬电催化剂
与常规的Haber-Bosch工艺相比,通过氮还原反应(NRR)进行氨的电化学合成作为一种对环境更友好,能耗更低的技术受到了广泛的关注。然而,迄今为止报道的所有NRR电催化剂的催化活性在环境条件下都非常低。在这项研究中,合成了部分氧化的氮化铬(氮氧化铬)纳米颗粒,并在环境条件下在质子交换膜电解槽中评估了其NRR活性。最高氨生成速率为8.9×10 -11 mol s -1 cm -2和15.56 µg h -1 mg -1猫 电池电压为2.0 V时可实现最高的法拉第效率。电池电压为1.8 V时可达到6.7%的最高法拉第效率。研究结果表明,基于金属氮化物的材料可以成为NRR的有希望的电催化剂,并可以指导更先进的材料的合理设计。各种反应的催化剂。
更新日期:2018-10-21
中文翻译:

常温条件下用于电化学合成氨的氧氮化铬电催化剂
与常规的Haber-Bosch工艺相比,通过氮还原反应(NRR)进行氨的电化学合成作为一种对环境更友好,能耗更低的技术受到了广泛的关注。然而,迄今为止报道的所有NRR电催化剂的催化活性在环境条件下都非常低。在这项研究中,合成了部分氧化的氮化铬(氮氧化铬)纳米颗粒,并在环境条件下在质子交换膜电解槽中评估了其NRR活性。最高氨生成速率为8.9×10 -11 mol s -1 cm -2和15.56 µg h -1 mg -1猫 电池电压为2.0 V时可实现最高的法拉第效率。电池电压为1.8 V时可达到6.7%的最高法拉第效率。研究结果表明,基于金属氮化物的材料可以成为NRR的有希望的电催化剂,并可以指导更先进的材料的合理设计。各种反应的催化剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号