当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of biotin-conjugated anticancer thiosemicarbazones and their iron(III) and copper(II) complexes.
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-10-19 , DOI: 10.1016/j.jinorgbio.2018.10.006 Sebastian Kallus 1 , Lukas Uhlik 2 , Sushilla van Schoonhoven 2 , Karla Pelivan 1 , Walter Berger 3 , Éva A Enyedy 4 , Thilo Hofmann 5 , Petra Heffeter 3 , Christian R Kowol 6 , Bernhard K Keppler 6
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-10-19 , DOI: 10.1016/j.jinorgbio.2018.10.006 Sebastian Kallus 1 , Lukas Uhlik 2 , Sushilla van Schoonhoven 2 , Karla Pelivan 1 , Walter Berger 3 , Éva A Enyedy 4 , Thilo Hofmann 5 , Petra Heffeter 3 , Christian R Kowol 6 , Bernhard K Keppler 6
Affiliation
|
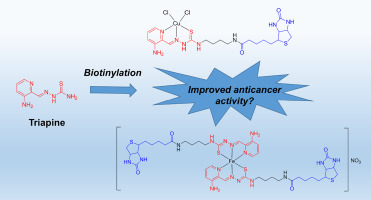
Triapine, the most prominent anticancer drug candidate from the substance class of thiosemicarbazones, was investigated in >30 clinical phase I and II studies. However, the results were rather disappointing against solid tumors, which can be explained (at least partially) due to inefficient delivery to the tumor site. Hence, we synthesized the first biotin-functionalized thiosemicarbazone derivatives in order to increase tumor specificity and accumulation. Additionally, for Triapine and one biotin conjugate the iron(III) and copper(II) complexes were prepared. Subsequently, the novel compounds were biologically evaluated on a cell line panel with different biotin uptake. The metal-free biotin-conjugated ligands showed comparable activity to the reference compound Triapine. However, astonishingly, the metal complexes of the biotinylated derivative showed strikingly decreased anticancer activity. To further analyze possible differences between the metal complexes, detailed physico- and electrochemical experiments were performed. However, neither lipophilicity or complex solution stability, nor the reduction potential or behavior in the presence of biologically relevant reducing agents showed strong variations between the biotinylated and non-biotinylated derivatives (only some differences in the reduction kinetics were observed). Nonetheless, the metal-free biotin-conjugate of Triapine revealed distinct activity in a colon cancer mouse model upon oral application comparable to Triapine. Therefore, this type of biotin-conjugated thiosemicarbazone is of interest for further synthetic strategies and biological studies.
中文翻译:

生物素结合的抗癌缩氨基硫脲及其铁(III)和铜(II)络合物的合成和生物学评价。
三甲平是缩氨基硫脲类物质中最著名的抗癌候选药物,在 >30 临床 I 期和 II 期研究中进行了研究。然而,针对实体瘤的结果相当令人失望,这可以(至少部分地)归因于肿瘤部位的递送效率低下。因此,我们合成了第一个生物素功能化缩氨基硫脲衍生物,以提高肿瘤特异性和积累。此外,对于三甲平和一种生物素缀合物,制备了铁(III)和铜(II)络合物。随后,在具有不同生物素摄取的细胞系组上对这些新型化合物进行了生物学评估。无金属生物素缀合配体显示出与参考化合物三甲平相当的活性。然而,令人惊讶的是,生物素化衍生物的金属配合物显示出显着降低的抗癌活性。为了进一步分析金属配合物之间可能存在的差异,进行了详细的物理和电化学实验。然而,生物素化和非生物素化衍生物之间的亲脂性或复杂溶液稳定性,以及在生物相关还原剂存在下的还原电位或行为都没有表现出强烈的差异(仅观察到还原动力学的一些差异)。尽管如此,三甲平的无金属生物素缀合物在口服给药后在结肠癌小鼠模型中显示出与三甲平相当的独特活性。因此,这种类型的生物素缀合缩氨基硫脲对于进一步的合成策略和生物学研究很有意义。
更新日期:2018-10-19
中文翻译:

生物素结合的抗癌缩氨基硫脲及其铁(III)和铜(II)络合物的合成和生物学评价。
三甲平是缩氨基硫脲类物质中最著名的抗癌候选药物,在 >30 临床 I 期和 II 期研究中进行了研究。然而,针对实体瘤的结果相当令人失望,这可以(至少部分地)归因于肿瘤部位的递送效率低下。因此,我们合成了第一个生物素功能化缩氨基硫脲衍生物,以提高肿瘤特异性和积累。此外,对于三甲平和一种生物素缀合物,制备了铁(III)和铜(II)络合物。随后,在具有不同生物素摄取的细胞系组上对这些新型化合物进行了生物学评估。无金属生物素缀合配体显示出与参考化合物三甲平相当的活性。然而,令人惊讶的是,生物素化衍生物的金属配合物显示出显着降低的抗癌活性。为了进一步分析金属配合物之间可能存在的差异,进行了详细的物理和电化学实验。然而,生物素化和非生物素化衍生物之间的亲脂性或复杂溶液稳定性,以及在生物相关还原剂存在下的还原电位或行为都没有表现出强烈的差异(仅观察到还原动力学的一些差异)。尽管如此,三甲平的无金属生物素缀合物在口服给药后在结肠癌小鼠模型中显示出与三甲平相当的独特活性。因此,这种类型的生物素缀合缩氨基硫脲对于进一步的合成策略和生物学研究很有意义。










































 京公网安备 11010802027423号
京公网安备 11010802027423号