Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Worm‐Like Biomimetic Nanoerythrocyte Carrying siRNA for Melanoma Gene Therapy
Small ( IF 13.0 ) Pub Date : 2018-10-17 , DOI: 10.1002/smll.201803002 Yanming Wang 1 , Xin Ji 1 , Miaoliang Ruan 1 , Wen Liu 1 , Rongguang Song 1 , Jian Dai 1 , Wei Xue 1, 2, 3
Small ( IF 13.0 ) Pub Date : 2018-10-17 , DOI: 10.1002/smll.201803002 Yanming Wang 1 , Xin Ji 1 , Miaoliang Ruan 1 , Wen Liu 1 , Rongguang Song 1 , Jian Dai 1 , Wei Xue 1, 2, 3
Affiliation
|
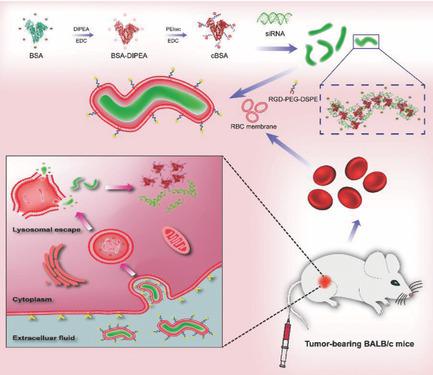
A major challenge in siRNA vectors is developing approaches that ensure that when administered in vivo, the vectors can target their requisite site of action. This study reports a third type of nanoworm, biomimetic nanoerythrocytes for siRNA delivery, except for filomicelle and nanoworm iron‐oxide particle, which is the first approach that allows for targeted siRNA delivery by a process involving red blood cell (RBC) membrane cloaking of charge‐reversible polyplexes of siRNA and polycation. RBC membrane cloaking protects siRNA from RNase A degradation. Moreover, the RBC membrane‐cloaked charge‐reversible siRNA vector (RBC‐reversible polyplex (RP)) not only stays longer in the blood circulation than that of negatively charged bovine serum albumin (BSA) spheres and positively charged BSA, but is also able to escape from late endosomes/lysosomes, to achieve effective transfection for gene knockdown. The knockdown result in vivo is remarkably consistent with that of intracellular trafficking and transfection in vitro. Due to the outstanding biocompatibility and active targeting (cRGD), the 7 mg kg−1 dose siSurvivin in RGD‐RBC‐RP exhibits obviously superior anticancer effects at the animal level after two weeks. Therefore, the biomimetic worm‐like nanoerythrocyte charge‐reversible gene vector is a new and general method for highly efficient siRNA therapy in vivo.
中文翻译:

蠕虫样仿生纳米红细胞携带siRNA用于黑色素瘤基因治疗
siRNA载体的主要挑战是开发确保在体内给药时载体可以靶向其必要的作用位点的方法。这项研究报告了第三种类型的siRNA递送纳米蠕虫,仿生纳米红细胞,除了细线虫和纳米蠕虫的氧化铁颗粒外,这是第一种允许通过涉及红细胞(RBC)膜掩盖电荷的过程靶向siRNA递送的方法。 siRNA和聚阳离子的可逆多链体。RBC膜掩盖保护siRNA免受RNase A降解。此外,RBC膜掩盖的电荷可逆siRNA载体(RBC可逆多链体(RP))不仅在血液循环中的停留时间比带负电荷的牛血清白蛋白(BSA)和带正电荷的BSA的更长,而且还能够从后期的内体/溶酶体中逃脱,实现基因敲除的有效转染。体内的敲除结果与体外细胞内运输和转染的结果显着一致。由于具有出色的生物相容性和主动靶向性(cRGD),因此7毫克/千克两周后,RGD-RBC-RP中的-1剂量siSurvivin在动物水平上表现出明显优越的抗癌作用。因此,仿生蠕虫样纳米红细胞电荷可逆基因载体是体内高效siRNA治疗的一种新的通用方法。
更新日期:2018-10-17
中文翻译:

蠕虫样仿生纳米红细胞携带siRNA用于黑色素瘤基因治疗
siRNA载体的主要挑战是开发确保在体内给药时载体可以靶向其必要的作用位点的方法。这项研究报告了第三种类型的siRNA递送纳米蠕虫,仿生纳米红细胞,除了细线虫和纳米蠕虫的氧化铁颗粒外,这是第一种允许通过涉及红细胞(RBC)膜掩盖电荷的过程靶向siRNA递送的方法。 siRNA和聚阳离子的可逆多链体。RBC膜掩盖保护siRNA免受RNase A降解。此外,RBC膜掩盖的电荷可逆siRNA载体(RBC可逆多链体(RP))不仅在血液循环中的停留时间比带负电荷的牛血清白蛋白(BSA)和带正电荷的BSA的更长,而且还能够从后期的内体/溶酶体中逃脱,实现基因敲除的有效转染。体内的敲除结果与体外细胞内运输和转染的结果显着一致。由于具有出色的生物相容性和主动靶向性(cRGD),因此7毫克/千克两周后,RGD-RBC-RP中的-1剂量siSurvivin在动物水平上表现出明显优越的抗癌作用。因此,仿生蠕虫样纳米红细胞电荷可逆基因载体是体内高效siRNA治疗的一种新的通用方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号