当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-dependent conformational changes of a proximal [4Fe–4S] cluster in Hyb-type [NiFe]-hydrogenase to protect the active site from O2†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1039/c8cc06261g Noor Dina Muhd Noor 1, 2, 3, 4, 5 , Hiroaki Matsuura 1, 2, 3, 4, 5 , Koji Nishikawa 1, 2, 3, 4, 5 , Hulin Tai 5, 6, 7, 8, 9 , Shun Hirota 5, 6, 7, 8, 9 , Jaehyun Kim 1, 2, 3, 4, 5 , Jiyoung Kang 1, 2, 3, 4, 5 , Masaru Tateno 1, 2, 3, 4, 5 , Ki-Seok Yoon 5, 10, 11, 12, 13 , Seiji Ogo 5, 10, 11, 12, 13 , Shintaro Kubota 1, 2, 3, 4, 5 , Yasuhito Shomura 5, 6, 7, 8, 14 , Yoshiki Higuchi 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-10-17 00:00:00 , DOI: 10.1039/c8cc06261g Noor Dina Muhd Noor 1, 2, 3, 4, 5 , Hiroaki Matsuura 1, 2, 3, 4, 5 , Koji Nishikawa 1, 2, 3, 4, 5 , Hulin Tai 5, 6, 7, 8, 9 , Shun Hirota 5, 6, 7, 8, 9 , Jaehyun Kim 1, 2, 3, 4, 5 , Jiyoung Kang 1, 2, 3, 4, 5 , Masaru Tateno 1, 2, 3, 4, 5 , Ki-Seok Yoon 5, 10, 11, 12, 13 , Seiji Ogo 5, 10, 11, 12, 13 , Shintaro Kubota 1, 2, 3, 4, 5 , Yasuhito Shomura 5, 6, 7, 8, 14 , Yoshiki Higuchi 1, 2, 3, 4, 5
Affiliation
|
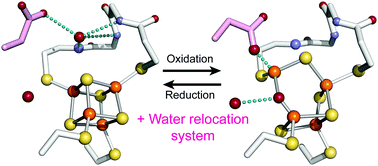
Citrobacter sp. S-77 [NiFe]-hydrogenase harbors a standard [4Fe–4S] cluster proximal to the Ni–Fe active site. The presence of relocatable water molecules and a flexible aspartate enables the [4Fe–4S] to display redox-dependent conformational changes. These structural features are proposed to be the key aspects that protect the active site from O2 attack.
中文翻译:

Hyb型[NiFe]氢化酶中近端[4Fe–4S]簇的氧化还原依赖性构象变化,以保护活性位点免受O 2 †
枸橼酸杆菌属。S-77 [NiFe]-加氢酶在Ni-Fe活性位点附近有一个标准的[4Fe-4S]簇。可重定位的水分子和灵活的天冬氨酸的存在使[4Fe-4S]能够显示出依赖于氧化还原的构象变化。这些结构特征被认为是保护活动站点免受O 2攻击的关键方面。
更新日期:2018-10-17
中文翻译:

Hyb型[NiFe]氢化酶中近端[4Fe–4S]簇的氧化还原依赖性构象变化,以保护活性位点免受O 2 †
枸橼酸杆菌属。S-77 [NiFe]-加氢酶在Ni-Fe活性位点附近有一个标准的[4Fe-4S]簇。可重定位的水分子和灵活的天冬氨酸的存在使[4Fe-4S]能够显示出依赖于氧化还原的构象变化。这些结构特征被认为是保护活动站点免受O 2攻击的关键方面。











































 京公网安备 11010802027423号
京公网安备 11010802027423号