当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiazolidine chemistry revisited: a fast, efficient and stable click-type reaction at physiological pH†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1039/c8cc05405c Daniel Bermejo-Velasco 1, 2, 3, 4, 5 , Ganesh N. Nawale 1, 2, 3, 4, 5 , Oommen P. Oommen 6, 7, 8, 9, 10 , Jöns Hilborn 1, 2, 3, 4, 5 , Oommen P. Varghese 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1039/c8cc05405c Daniel Bermejo-Velasco 1, 2, 3, 4, 5 , Ganesh N. Nawale 1, 2, 3, 4, 5 , Oommen P. Oommen 6, 7, 8, 9, 10 , Jöns Hilborn 1, 2, 3, 4, 5 , Oommen P. Varghese 1, 2, 3, 4, 5
Affiliation
|
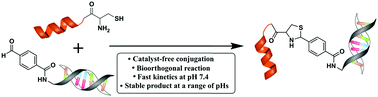
We describe the fast reaction kinetics between 1,2-aminothiols and aldehydes. Under physiological conditions such a click-type reaction afforded a thiazolidine product that remains stable and did not require any catalyst. This type of bioorthogonal reaction offers enormous potential for the coupling of biomolecules in an efficient and biocompatible manner.
中文翻译:

重审噻唑烷化学:在生理pH值下快速,有效和稳定的点击型反应†
我们描述了1,2-氨基硫醇和醛之间的快速反应动力学。在生理条件下,这种点击型反应提供了噻唑烷产物,该产物保持稳定并且不需要任何催化剂。这种类型的生物正交反应为以有效且生物相容的方式偶联生物分子提供了巨大的潜力。
更新日期:2018-10-16
中文翻译:

重审噻唑烷化学:在生理pH值下快速,有效和稳定的点击型反应†
我们描述了1,2-氨基硫醇和醛之间的快速反应动力学。在生理条件下,这种点击型反应提供了噻唑烷产物,该产物保持稳定并且不需要任何催化剂。这种类型的生物正交反应为以有效且生物相容的方式偶联生物分子提供了巨大的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号