International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2018-10-16 , DOI: 10.1016/j.ijhydene.2018.09.146 Kai Coenen , Fausto Gallucci , Emiel Hensen , Martin van Sint Annaland
|
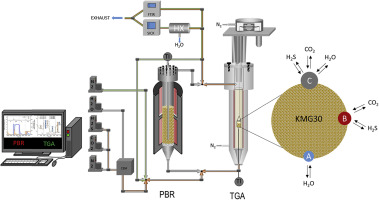
Adsorption of H2S and the influence of steam on its adsorption capacity and kinetics were studied on a commercial potassium-promoted hydrotalcite. The sorbent shows a very high cyclic working capacity for H2S compared to CO2 and H2O, even at lower partial pressures and at different operating temperatures ranging between 300 and 500 °C. The operating temperature does not significantly influence the cyclic working capacity for half-cycle times of 30 min. The adsorption mechanism, however, changes at higher temperatures. At lower temperatures (300 °C) a fast adsorption with a fast approach to steady state was observed. At higher operating temperatures, H2S reacts with the hydrotalcite structure, forming strongly bonded sulfuric species on the sorbent. When using dry regeneration conditions, the first cycles in cyclic operation at higher temperatures show a significantly higher adsorption of H2S (especially the first cycle), which cannot be desorbed during regeneration with N2. After the first fast initial adsorption rate a continuous slow adsorption of H2S occurs, probably caused by a surface reaction between H2S and the hydrotalcite structure. This reaction is, however, reversible if steam is used.
The adsorption mechanism for H2S and H2O was determined using multiple cyclic experiments comparable to previous studies performed for CO2 and H2O adsorption. It is evident that the adsorption mechanism developed for CO2 on the same sorbents is also valid for H2S, indicating that the developed mechanism is consistent for sour gas adsorption on this type of sorbents. The cyclic working capacity can be significantly increased if steam is used during the regeneration step of the sorbent. The mechanistic model developed for the adsorption of CO2 and H2O was successfully validated with more than 160 different TGA experiments. An operating temperature of 400 °C seems to be optimal to achieve a high cyclic working capacity for H2S, because at higher temperatures the regeneration of the formed sulfuric species seems to be hindered resulting in a significant decrease in the cyclic working capacity.
中文翻译:

H 2 S在钾促进的水滑石上的吸附行为和动力学
在市售钾促进的水滑石上研究了H 2 S的吸附以及蒸汽对其吸附能力和动力学的影响。与CO 2和H 2 O相比,即使在较低的分压和300至500°C的不同工作温度下,吸附剂对H 2 S仍具有非常高的循环工作能力。在30分钟的半循环时间内,工作温度不会显着影响循环工作能力。但是,吸附机理在较高温度下会发生变化。在较低的温度(300°C)下,观察到快速吸附并快速接近稳态。在较高的工作温度下,H 2S与水滑石结构反应,在吸附剂上形成牢固结合的硫物质。当使用干燥再生条件时,在较高温度下的循环操作中的第一个循环显示出明显更高的H 2 S吸附(尤其是第一个循环),而在用N 2再生期间不能解吸。在第一个快速的初始吸附速率之后,H 2 S连续缓慢地吸附,这可能是由于H 2 S与水滑石结构之间的表面反应引起的。但是,如果使用蒸汽,则该反应是可逆的。
使用与先前对CO 2和H 2 O吸附进行的研究相当的多个循环实验,确定了H 2 S和H 2 O的吸附机理。显然,在相同的吸附剂上针对CO 2形成的吸附机理对H 2 S也有效,这表明该机理对于酸性气体在该类型的吸附剂上的吸附是一致的。如果在吸附剂的再生步骤中使用蒸汽,循环工作能力会大大提高。建立了吸附CO 2和H 2的机理模型O已通过160多个不同的TGA实验成功验证。为了达到高的H 2 S循环工作能力,400°C的工作温度似乎是最佳的,因为在较高的温度下,似乎阻碍了所形成的硫酸类物质的再生,从而导致循环工作能力显着降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号