当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scoping the Enantioselective Desymmetrization of a Poorly Water-Soluble Diester by Recombinant Pig Liver Esterase
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1021/acs.oprd.8b00277 Murray P. Meissner 1, 2 , Philipp Süss 3 , Henrike Brundiek 3 , John M. Woodley 1 , Jan von Langermann 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1021/acs.oprd.8b00277 Murray P. Meissner 1, 2 , Philipp Süss 3 , Henrike Brundiek 3 , John M. Woodley 1 , Jan von Langermann 4
Affiliation
|
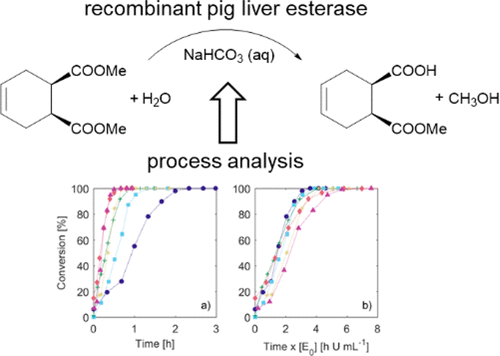
Previously, the biocatalytic desymmetrization of dimethyl cyclohex-4-ene-cis-1,2-dicarboxylate to (1S,2R)-1-(methoxycarbonyl)cyclohex-4-ene-2-carboxylic acid, an important intermediate toward the synthesis of biologically active molecules, had been well-characterized in terms of pH and temperature optima and several aspects of process performance. Eventually this promising reaction could convert 200 mM (40 g·L–1) of substrate with >99.5% ee using the recombinant pig liver esterase, ECS-PLE06, at a scale of 8.8 L. However, the precise influence of substrate concentration and the poorly water-soluble nature of the substrate (approximately 60 mM in water at 25 °C for structurally similar dimethyl 1,4-cyclohexanedicarboxylate) remained elusive. Therefore, this work focuses on using a recently published methodology based on reaction trajectory analysis to identify mass transfer limitations in this reaction. With the constraints of mass transfer on space-time yield considered, it was possible to evaluate and improve biocatalyst yield (mass of product per mass of biocatalyst) through the use of higher substrate concentrations. Ultimately the complete conversion of approximately 75 g·L–1 substrate was achieved in 3.65 h yielding an excellent productivity of 20 g·L–1·h–1 with a biocatalyst yield of 4.36 g·gbiocat–1. This work also highlights the simplicity of applying a reaction trajectory analysis methodology and the importance of scale during reaction characterizations, and it identifies future directions for reaction improvement to address substrate supply and product inhibition/deactivation.
中文翻译:

重组猪肝酯酶确定水溶性差的二酯的对映选择性不对称化
以前,二甲基环己-4-烯-顺式-1,2-二羧酸酯的生物催化脱对称化为(1 S,2 R)-1-(甲氧基羰基)环己-4-烯-2-羧酸的重要中间体。 pH和温度的最佳状态以及工艺性能的几个方面,已经很好地描述了生物活性分子的合成。最终,这种有希望的反应可以转化为200 mM(40 g·L –1),使用重组猪肝酯酶ECS-PLE06,在8.8 L的范围内分离出> 99.5%ee的底物。但是,底物浓度的精确影响和底物的水溶性差(在水中约60 mM)在25°C下,对于结构相似的1,4-环己烷二甲酸二甲酯)仍然难以捉摸。因此,这项工作着重于使用基于反应轨迹分析的最新发表的方法来确定该反应中的传质限制。考虑到传质对时空产率的限制,可以通过使用较高的底物浓度来评估和提高生物催化剂的产率(每质量生物催化剂的产物质量)。最终完成约75 g·L –1的完全转化在3.65 h内获得了底物,产生了20 g·L –1 ·h –1的优异生产率,生物催化剂产量为4.36 g•g biocat –1。这项工作还强调了应用反应轨迹分析方法的简便性以及在反应表征过程中规模化的重要性,并且它确定了反应改进的未来方向,以解决底物供应和产物抑制/失活问题。
更新日期:2018-10-16
中文翻译:

重组猪肝酯酶确定水溶性差的二酯的对映选择性不对称化
以前,二甲基环己-4-烯-顺式-1,2-二羧酸酯的生物催化脱对称化为(1 S,2 R)-1-(甲氧基羰基)环己-4-烯-2-羧酸的重要中间体。 pH和温度的最佳状态以及工艺性能的几个方面,已经很好地描述了生物活性分子的合成。最终,这种有希望的反应可以转化为200 mM(40 g·L –1),使用重组猪肝酯酶ECS-PLE06,在8.8 L的范围内分离出> 99.5%ee的底物。但是,底物浓度的精确影响和底物的水溶性差(在水中约60 mM)在25°C下,对于结构相似的1,4-环己烷二甲酸二甲酯)仍然难以捉摸。因此,这项工作着重于使用基于反应轨迹分析的最新发表的方法来确定该反应中的传质限制。考虑到传质对时空产率的限制,可以通过使用较高的底物浓度来评估和提高生物催化剂的产率(每质量生物催化剂的产物质量)。最终完成约75 g·L –1的完全转化在3.65 h内获得了底物,产生了20 g·L –1 ·h –1的优异生产率,生物催化剂产量为4.36 g•g biocat –1。这项工作还强调了应用反应轨迹分析方法的简便性以及在反应表征过程中规模化的重要性,并且它确定了反应改进的未来方向,以解决底物供应和产物抑制/失活问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号