当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of a Practical Enzymatic Synthesis of Isosorbide-2-acetate
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1021/acs.oprd.8b00310 Shi-Guo Zhu 1 , Jia-Xin Huang 2 , Gui-Min Zhang 3 , Shao-Xin Chen 4 , Fu-Li Zhang 1, 4
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-10-16 00:00:00 , DOI: 10.1021/acs.oprd.8b00310 Shi-Guo Zhu 1 , Jia-Xin Huang 2 , Gui-Min Zhang 3 , Shao-Xin Chen 4 , Fu-Li Zhang 1, 4
Affiliation
|
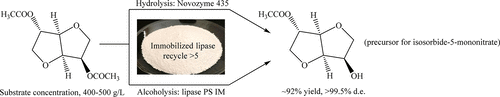
Using immobilized lipase, we developed an enzymatic synthesis of isosorbide-2-acetate (1), a key intermediate in the preparation of isosorbide-5-mononitrate. 1 was prepared in high yield (∼92%) with excellent regioselectivity (>99.5% d.e.) through hydrolysis (Novozym 435) or alcoholysis (lipase PS IM) of isosorbide-2,5-diacetate. The conditions for the enzymatic process were optimized. The substrate concentration could be 400–500 g/L, and the enzyme to substrate ratio was <10 wt %. The feasibility of enzyme recycling was also demonstrated. In addition, the enzymatic process was carried out on a kilogram scale and was proved to be scalable, efficient, and economical.
中文翻译:

实用的酶法合成2-乙酸异山梨酯的进展
使用固定化的脂肪酶,我们开发了异山梨醇-2-乙酸酯的酶促合成方法(1),这是制备异山梨醇-5-单硝酸酯的关键中间体。1在高收率(〜92%)与异山梨醇-2,5-二乙酸酯的优良的区位选择性(> 99.5%DE)通过水解(诺维信435)或醇(脂肪酶PS IM)制备。优化了酶促工艺的条件。底物浓度可能为400–500 g / L,酶与底物的比例为<10 wt%。还证明了酶循环的可行性。另外,酶促过程以千克规模进行,并且被证明是可扩展的,有效的和经济的。
更新日期:2018-10-16
中文翻译:

实用的酶法合成2-乙酸异山梨酯的进展
使用固定化的脂肪酶,我们开发了异山梨醇-2-乙酸酯的酶促合成方法(1),这是制备异山梨醇-5-单硝酸酯的关键中间体。1在高收率(〜92%)与异山梨醇-2,5-二乙酸酯的优良的区位选择性(> 99.5%DE)通过水解(诺维信435)或醇(脂肪酶PS IM)制备。优化了酶促工艺的条件。底物浓度可能为400–500 g / L,酶与底物的比例为<10 wt%。还证明了酶循环的可行性。另外,酶促过程以千克规模进行,并且被证明是可扩展的,有效的和经济的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号