当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitration of amyloid-β peptide (1-42) as a protective mechanism for the amyloid-β peptide (1-42) against copper ion toxicity.
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-10-16 , DOI: 10.1016/j.jinorgbio.2018.10.005 Jie Zhao 1 , Wanxia Gao 2 , Zhen Yang 3 , Hailing Li 1 , Zhonghong Gao 1
Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2018-10-16 , DOI: 10.1016/j.jinorgbio.2018.10.005 Jie Zhao 1 , Wanxia Gao 2 , Zhen Yang 3 , Hailing Li 1 , Zhonghong Gao 1
Affiliation
|
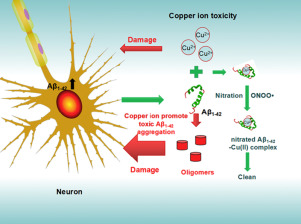
It is known that copper ion (Cu(II)) binds to amyloid-β peptide (Aβ), induces Aβ oligomer formation and ultimately exacerbates Aβ-aggregation neurotoxicity in Alzheimer's disease (AD). It becomes interesting to know that how this chemical modification of Aβ would affect interaction of Aβ and Cu(II) and their roles in the development of AD. In this work, we investigated the interaction of Aβ1-42 nitration with the toxic Cu(II). It showed that Cu(II)induced Aβ1-42 nitration in the presence of nitrite and hydrogen peroxide. Circular dichroism studies also revealed significant conformational change of Aβ1-42 and Tyr10 nitrated amyloid-β peptide(1-42) (Aβ1-42NT) when interacting with Cu(II). Even though nitration did not alter the binding of Aβ1-42 to Cu(II) or the peroxidative activity of Aβ1-42-Cu(II) complex, nitration ameliorated the aggregation and neurotoxicity of Aβ1-42 induced by Cu(II), which was also further confirmed by the cell study. Given our previous findings that Aβ nitration dramatically inhibited its aggregation and thus reduced its toxicity, we speculated that nitration of Aβ1-42 altered its intermolecular interaction, which protected itself against the toxicity of Cu(II). Based on this hypothesis, we propose that nitration of Aβ1-42 may be an important protective mechanism for normal function of Aβ1-42 and deserves more attention in AD drug development.
中文翻译:

硝化β-淀粉样肽(1-42)作为β-淀粉样肽(1-42)抵抗铜离子毒性的保护机制。
已知铜离子(Cu(II))与淀粉样β肽(Aβ)结合,诱导Aβ低聚物形成,并最终加剧阿尔茨海默氏病(AD)中的Aβ聚集神经毒性。有趣的是,这种Aβ的化学修饰将如何影响Aβ和Cu(II)的相互作用及其在AD发生中的作用。在这项工作中,我们研究了Aβ1-42硝化与有毒Cu(II)的相互作用。结果表明,在亚硝酸盐和过氧化氢存在下,Cu(II)诱导了Aβ1-42的硝化。圆二色性研究还显示,当与Cu(II)相互作用时,Aβ1-42和Tyr10硝化淀粉样β-肽(1-42)(Aβ1-42NT)的构象发生了显着变化。即使硝化作用不会改变Aβ1-42与Cu(II)的结合或Aβ1-42-Cu(II)复合物的过氧化活性,硝化作用改善了Cu(II)诱导的Aβ1-42的聚集和神经毒性,细胞研究也进一步证实了这一点。鉴于我们先前的发现,Aβ硝化显着抑制了其聚集并因此降低了其毒性,我们推测Aβ1-42的硝化改变了其分子间的相互作用,从而保护了自身免受Cu(II)的毒性。基于该假设,我们认为硝化Aβ1-42可能是Aβ1-42正常功能的重要保护机制,在AD药物开发中应引起更多关注。我们推测,硝化Aβ1-42会改变其分子间相互作用,从而保护自己免受Cu(II)的毒性。基于该假设,我们认为硝化Aβ1-42可能是Aβ1-42正常功能的重要保护机制,在AD药物开发中应引起更多关注。我们推测,硝化Aβ1-42会改变其分子间相互作用,从而保护自己免受Cu(II)的毒性。基于该假设,我们认为硝化Aβ1-42可能是Aβ1-42正常功能的重要保护机制,在AD药物开发中应引起更多关注。
更新日期:2018-10-16
中文翻译:

硝化β-淀粉样肽(1-42)作为β-淀粉样肽(1-42)抵抗铜离子毒性的保护机制。
已知铜离子(Cu(II))与淀粉样β肽(Aβ)结合,诱导Aβ低聚物形成,并最终加剧阿尔茨海默氏病(AD)中的Aβ聚集神经毒性。有趣的是,这种Aβ的化学修饰将如何影响Aβ和Cu(II)的相互作用及其在AD发生中的作用。在这项工作中,我们研究了Aβ1-42硝化与有毒Cu(II)的相互作用。结果表明,在亚硝酸盐和过氧化氢存在下,Cu(II)诱导了Aβ1-42的硝化。圆二色性研究还显示,当与Cu(II)相互作用时,Aβ1-42和Tyr10硝化淀粉样β-肽(1-42)(Aβ1-42NT)的构象发生了显着变化。即使硝化作用不会改变Aβ1-42与Cu(II)的结合或Aβ1-42-Cu(II)复合物的过氧化活性,硝化作用改善了Cu(II)诱导的Aβ1-42的聚集和神经毒性,细胞研究也进一步证实了这一点。鉴于我们先前的发现,Aβ硝化显着抑制了其聚集并因此降低了其毒性,我们推测Aβ1-42的硝化改变了其分子间的相互作用,从而保护了自身免受Cu(II)的毒性。基于该假设,我们认为硝化Aβ1-42可能是Aβ1-42正常功能的重要保护机制,在AD药物开发中应引起更多关注。我们推测,硝化Aβ1-42会改变其分子间相互作用,从而保护自己免受Cu(II)的毒性。基于该假设,我们认为硝化Aβ1-42可能是Aβ1-42正常功能的重要保护机制,在AD药物开发中应引起更多关注。我们推测,硝化Aβ1-42会改变其分子间相互作用,从而保护自己免受Cu(II)的毒性。基于该假设,我们认为硝化Aβ1-42可能是Aβ1-42正常功能的重要保护机制,在AD药物开发中应引起更多关注。











































 京公网安备 11010802027423号
京公网安备 11010802027423号