International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2018-10-15 , DOI: 10.1016/j.ijhydene.2018.09.087 Luis A. Romero-Cano , G. Rosado-Ortiz , A.M. Valenzuela-Muñiz , L.C. Ordóñez , R. Gauvin , Y. Verde Gómez
|
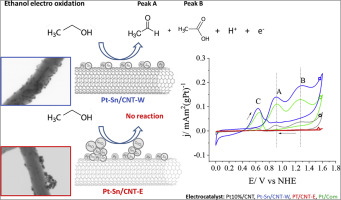
Pt and Pt–Sn nanoparticles were synthesized and supported onto carbon nanotubes (CNT), the electrocatalytic activity towards the ethanol oxidation reaction was analyzed. The effect of the solvent employed for the synthesis was evaluated. Metal nanoparticles synthesis was made using water (Pt–Sn/CNT-W) or ethanol (Pt–Sn/CNT-E) as a solvent. Pt–Sn/CNT-W material presented only Pt–Sn alloy nanoparticles homogeneously distributed on the carbon nanotubes support. Pt–Sn/CNT-E sample showed non well-dispersed nanoparticles forming agglomerates along the CNTs surface with predominantly Sn4+ superficial species (SnO2) as show the XPS, FTIR and electrochemical results. These surface arrangements had important effects on the electrocatalytic properties. Pt–Sn/CNT-W shows higher ethanol electrooxidation activity than the Pt–Sn/CNT-E, which is attributed to: a) the double catalytic effect and the intrinsic electronic mechanism favored by the presence of Sn; b) the good particle dispersion of the bimetallic active phase on the CNT and; c) the absence of SnO2 species.
中文翻译:

纳米结构Pt-Sn / CNT合成中的溶剂效应作为乙醇电氧化的电催化剂
合成了Pt和Pt-Sn纳米颗粒并将其负载在碳纳米管(CNT)上,分析了其对乙醇氧化反应的电催化活性。评价了用于合成的溶剂的效果。金属纳米颗粒的合成是用水(Pt-Sn / CNT-W)或乙醇(Pt-Sn / CNT-E)作为溶剂进行的。Pt-Sn / CNT-W材料仅呈现均匀分布在碳纳米管载体上的Pt-Sn合金纳米颗粒。Pt-Sn / CNT-E样品显示出未充分分散的纳米颗粒沿着CNTs表面形成附聚物,主要为Sn 4+表层物质(SnO 2),如显示XPS,FTIR和电化学结果。这些表面排列对电催化性能具有重要影响。Pt-Sn / CNT-W比Pt-Sn / CNT-E表现出更高的乙醇电氧化活性,这归因于:a)锡的存在有利于双重催化作用和固有的电子机理;b)双金属活性相在CNT上的良好颗粒分散性;以及 c)不存在SnO 2物种。











































 京公网安备 11010802027423号
京公网安备 11010802027423号