当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tumor-Acidity-Cleavable Maleic Acid Amide (TACMAA): A Powerful Tool for Designing Smart Nanoparticles To Overcome Delivery Barriers in Cancer Nanomedicine
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-10-15 00:00:00 , DOI: 10.1021/acs.accounts.8b00195 Jin-Zhi Du 1, 2, 3 , Hong-Jun Li 2, 4 , Jun Wang 1, 3, 5, 6
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-10-15 00:00:00 , DOI: 10.1021/acs.accounts.8b00195 Jin-Zhi Du 1, 2, 3 , Hong-Jun Li 2, 4 , Jun Wang 1, 3, 5, 6
Affiliation
|
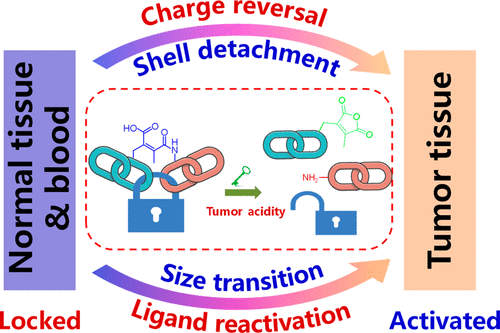
Over the past few decades, cancer nanomedicine has been under intensive development for applications in drug delivery, cancer therapy, and molecular imaging. However, there exist a series of complex biological barriers in the path of a nanomedicine from the site of administration to the site of action. These barriers considerably prevent a nanomedicine from reaching its targets in a sufficient concentration and thus severely limit its therapeutic benefits. According to the delivery process, these biological delivery barriers can be briefly summarized in the following order: blood circulation, tumor accumulation, tumor penetration, cellular internalization, and intracellular drug release. The therapeutic effect of a nanomedicine is strongly determined by its ability to overcome these barriers. However, advances in cancer biology have revealed that each barrier has its own distinct microenvironment, which imposes different requirements on the optimal design of nanocarriers, thus further complicating the delivery process. For example, the pH of blood is neutral, while the tumor extracellular environment features an acidic pH (pHe ≈ 6.5–7.0) and the endosome and lysosome are more acidic (pH 5.5–4.5). The nanoparticles (NPs) should be able to change their properties to adapt to each individual environment for robust and effective delivery. This demand promotes the design and development of smart delivery carriers that can respond to endogenous and exogenous stimuli.
中文翻译:

肿瘤酸可裂解的顺丁烯二酰胺(TACMAA):一个强大的工具,用于设计智能纳米颗粒以克服癌症纳米医学中的递送障碍。
在过去的几十年中,癌症纳米医学一直在深入开发,以用于药物输送,癌症治疗和分子成像。然而,在纳米药物从给药部位到作用部位的路径中存在一系列复杂的生物屏障。这些屏障极大地阻止了纳米药物以足够的浓度到达其靶标,因此严重限制了其治疗益处。根据传递过程,可以按以下顺序简要概述这些生物传递障碍:血液循环,肿瘤蓄积,肿瘤渗透,细胞内在化和细胞内药物释放。纳米药物的治疗效果在很大程度上取决于其克服这些障碍的能力。然而,癌症生物学的进步表明,每个屏障都有自己独特的微环境,这对纳米载体的优化设计提出了不同的要求,从而使递送过程更加复杂。例如,血液的pH值是中性的,而肿瘤细胞外环境具有酸性pH值(pHÈ ≈6.5-7.0)和核内体和溶酶体更酸性的(pH值5.5-4.5)。纳米颗粒(NPs)应该能够改变其性能,以适应每个单独的环境,以实现稳固而有效的输送。这种需求促进了可以响应内源性和外源性刺激的智能传递载体的设计和开发。
更新日期:2018-10-15
中文翻译:

肿瘤酸可裂解的顺丁烯二酰胺(TACMAA):一个强大的工具,用于设计智能纳米颗粒以克服癌症纳米医学中的递送障碍。
在过去的几十年中,癌症纳米医学一直在深入开发,以用于药物输送,癌症治疗和分子成像。然而,在纳米药物从给药部位到作用部位的路径中存在一系列复杂的生物屏障。这些屏障极大地阻止了纳米药物以足够的浓度到达其靶标,因此严重限制了其治疗益处。根据传递过程,可以按以下顺序简要概述这些生物传递障碍:血液循环,肿瘤蓄积,肿瘤渗透,细胞内在化和细胞内药物释放。纳米药物的治疗效果在很大程度上取决于其克服这些障碍的能力。然而,癌症生物学的进步表明,每个屏障都有自己独特的微环境,这对纳米载体的优化设计提出了不同的要求,从而使递送过程更加复杂。例如,血液的pH值是中性的,而肿瘤细胞外环境具有酸性pH值(pHÈ ≈6.5-7.0)和核内体和溶酶体更酸性的(pH值5.5-4.5)。纳米颗粒(NPs)应该能够改变其性能,以适应每个单独的环境,以实现稳固而有效的输送。这种需求促进了可以响应内源性和外源性刺激的智能传递载体的设计和开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号