当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Z-Selective Addition of Diaryl Phosphine Oxides to Alkynes via Photoredox Catalysis
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-15 00:00:00 , DOI: 10.1021/acscatal.8b02617 Huamin Wang 1 , Yongli Li 1 , Zilu Tang 1 , Shengchun Wang 1 , Heng Zhang 1 , Hengjiang Cong 1 , Aiwen Lei 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-15 00:00:00 , DOI: 10.1021/acscatal.8b02617 Huamin Wang 1 , Yongli Li 1 , Zilu Tang 1 , Shengchun Wang 1 , Heng Zhang 1 , Hengjiang Cong 1 , Aiwen Lei 1, 2
Affiliation
|
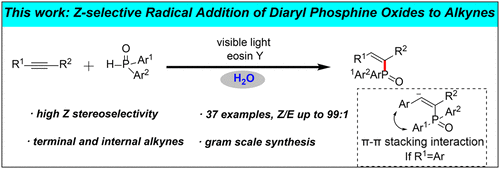
Radical addition to alkynes is known to predominantly yield thermodynamically more stable E-alkenes. Control of stereoselectivity in these reactions, and the isolation of the higher-energy Z-alkenes, remain an important challenge in chemical synthesis. Herein, direct synthesis of Z-alkenylphosphine oxides via visible-light-induced radical addition to alkynes in water is reported. This protocol was effective with various terminal and internal alkynes, affording products with high Z-stereoselectivity. Moreover, this transformation was demonstrated on gram scale. Mechanistic studies support the following conclusions: (1) the reaction proceeds via free-radical addition; (2) the choice of K2CO3 as aqueous base is crucial to the transformation; and (3) π–π stacking interaction greatly improves Z-selectivity.
中文翻译:

Z-选择性地通过光氧化还原催化将二芳基氧化膦加成至炔烃
已知向炔烃中自由基加成主要产生热力学上更稳定的E-烯烃。这些反应中立体选择性的控制以及高能Z-烯烃的分离仍然是化学合成中的重要挑战。本文中,报道了通过可见光诱导的自由基加成至水中的炔烃直接合成Z-烯基膦氧化物。该方案对各种末端和内部炔烃均有效,可提供具有高Z-立体选择性的产物。此外,这种转化以克为单位进行了证明。机理研究支持以下结论:(1)通过自由基加成反应。(2)K 2 CO 3的选择因为水性碱对转化至关重要;(3)π–π堆积相互作用极大地提高了Z选择性。
更新日期:2018-10-15
中文翻译:

Z-选择性地通过光氧化还原催化将二芳基氧化膦加成至炔烃
已知向炔烃中自由基加成主要产生热力学上更稳定的E-烯烃。这些反应中立体选择性的控制以及高能Z-烯烃的分离仍然是化学合成中的重要挑战。本文中,报道了通过可见光诱导的自由基加成至水中的炔烃直接合成Z-烯基膦氧化物。该方案对各种末端和内部炔烃均有效,可提供具有高Z-立体选择性的产物。此外,这种转化以克为单位进行了证明。机理研究支持以下结论:(1)通过自由基加成反应。(2)K 2 CO 3的选择因为水性碱对转化至关重要;(3)π–π堆积相互作用极大地提高了Z选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号