当前位置:
X-MOL 学术
›
Acta Pharmacol. Sin.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple circulating saponins from intravenous ShenMai inhibit OATP1Bs in vitro: potential joint precipitants of drug interactions.
Acta Pharmacologica Sinica ( IF 6.9 ) Pub Date : 2018-10-16 , DOI: 10.1038/s41401-018-0173-9 Olajide E Olaleye 1, 2 , Wei Niu 1 , Fei-Fei Du 1 , Feng-Qing Wang 1 , Fang Xu 1 , Salisa Pintusophon 1 , Jun-Lan Lu 1 , Jun-Ling Yang 1 , Chuan Li 1, 2
Acta Pharmacologica Sinica ( IF 6.9 ) Pub Date : 2018-10-16 , DOI: 10.1038/s41401-018-0173-9 Olajide E Olaleye 1, 2 , Wei Niu 1 , Fei-Fei Du 1 , Feng-Qing Wang 1 , Fang Xu 1 , Salisa Pintusophon 1 , Jun-Lan Lu 1 , Jun-Ling Yang 1 , Chuan Li 1, 2
Affiliation
|
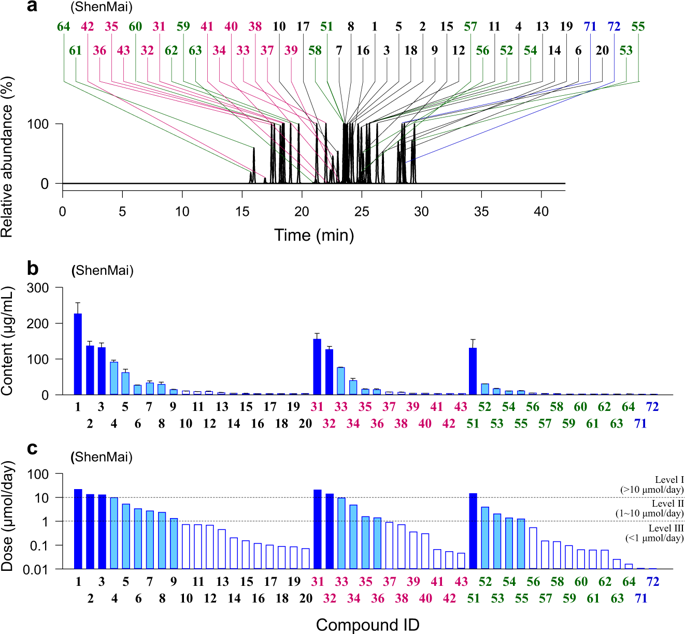
ShenMai, an intravenous injection prepared from steamed Panax ginseng roots (Hongshen) and Ophiopogon japonicus roots (Maidong), is used as an add-on therapy for coronary artery disease and cancer; saponins are its bioactive constituents. Since many saponins inhibit human organic anion-transporting polypeptides (OATP)1B, this investigation determined the inhibition potencies of circulating ShenMai saponins on the transporters and the joint potential of these compounds for ShenMai-drug interaction. Circulating saponins and their pharmacokinetics were characterized in rats receiving a 30-min infusion of ShenMai at 10 mL/kg. Inhibition of human OATP1B1/1B3 and rat Oatp1b2 by the individual saponins was investigated in vitro; the compounds' joint inhibition was also assessed in vitro and the data was processed using the Chou-Talalay method. Plasma protein binding was assessed by equilibrium dialysis. Altogether, 49 saponins in ShenMai were characterized and graded into: 10-100 μmol/day (compound doses from ShenMai; 7 compounds), 1-10 μmol/day (17 compounds), and <1 μmol/day (25 compounds, including Maidong ophiopogonins). After dosing, circulating saponins were protopanaxadiol-type ginsenosides Rb1, Rb2, Rc, Rd, Ra1, Rg3, Ra2, and Ra3, protopanaxatriol-type ginsenosides Rg1, Re, Rg2, and Rf, and ginsenoside Ro. The protopanaxadiol-type ginsenosides exhibited maximum plasma concentrations of 2.1-46.6 μmol/L, plasma unbound fractions of 0.4-1.0% and terminal half-lives of 15.6-28.5 h (ginsenoside Rg3, 1.9 h), while the other ginsenosides exhibited 0.1-7.7 μmol/L, 20.8-99.2%, and 0.2-0.5 h, respectively. The protopanaxadiol-type ginsenosides, ginsenosides without any sugar attachment at C-20 (except ginsenoside Rf), and ginsenoside Ro inhibited OATP1B3 more potently (IC50, 0.2-3.5 µmol/L) than the other ginsenosides (≥22.6 µmol/L). Inhibition of OATP1B1 by ginsenosides was less potent than OATP1B3 inhibition. Ginsenosides Rb1, Rb2, Rc, Rd, Ro, Ra1, Re, and Rg2 likely contribute the major part of OATP1B3-mediated ShenMai-drug interaction potential, in an additive and time-related manner.
中文翻译:

静脉注射参麦中的多种循环皂苷在体外抑制OATP1Bs:药物相互作用的潜在联合沉淀剂。
参麦是由蒸三七人参根(红参)和麦冬(麦冬)制成的静脉注射剂,可用于治疗冠状动脉疾病和癌症。皂苷是其生物活性成分。由于许多皂苷可抑制人有机阴离子转运多肽(OATP)1B,因此本研究确定了循环中的神麦皂苷对转运蛋白的抑制能力以及这些化合物与神麦-药物相互作用的联合潜力。在以10 mL / kg的剂量接受30分钟的深麦注射液的大鼠中,对循环皂苷及其药代动力学进行了表征。体外研究了单个皂苷对人OATP1B1 / 1B3和大鼠Oatp1b2的抑制作用。还评估了化合物的联合抑制作用,并使用Chou-Talalay方法处理了数据。通过平衡透析评估血浆蛋白结合。总共对沉麦中的49种皂苷进行了表征,并将其分为:10-100μmol/天(来自ShenMai的化合物剂量; 7种化合物),1-10μmol/天(17种化合物)和<1μmol/天(25种化合物,包括麦冬麦草碱)。给药后,循环的皂苷为原人参二醇型人参皂苷Rb1,Rb2,Rc,Rd,Ra1,Rg3,Ra2和Ra3,原人参三醇型人参皂苷Rg1,Re,Rg2和Rf以及人参皂苷Ro。原人参二醇型人参皂苷最大血浆浓度为2.1-46.6μmol/ L,血浆未结合分数为0.4-1.0%,终末半衰期为15.6-28.5 h(人参皂苷Rg3为1.9 h),而其他人参皂苷则为0.1-分别为7.7μmol/ L,20.8-99.2%和0.2-0.5 h。原人参二醇型人参皂甙,在C-20处没有任何糖附着的人参皂甙(人参皂甙Rf除外),人参皂甙Ro对OATP1B3的抑制作用(IC50,0.2-3.5 µmol / L)比其他人参皂甙(≥22.6µmol / L)更有力。人参皂甙对OATP1B1的抑制作用不如对OATP1B3的抑制作用强。人参皂苷Rb1,Rb2,Rc,Rd,Ro,Ra1,Re和Rg2可能以加成和时间相关的方式贡献了OATP1B3介导的深麦与药物相互作用的主要部分。
更新日期:2019-01-26
中文翻译:

静脉注射参麦中的多种循环皂苷在体外抑制OATP1Bs:药物相互作用的潜在联合沉淀剂。
参麦是由蒸三七人参根(红参)和麦冬(麦冬)制成的静脉注射剂,可用于治疗冠状动脉疾病和癌症。皂苷是其生物活性成分。由于许多皂苷可抑制人有机阴离子转运多肽(OATP)1B,因此本研究确定了循环中的神麦皂苷对转运蛋白的抑制能力以及这些化合物与神麦-药物相互作用的联合潜力。在以10 mL / kg的剂量接受30分钟的深麦注射液的大鼠中,对循环皂苷及其药代动力学进行了表征。体外研究了单个皂苷对人OATP1B1 / 1B3和大鼠Oatp1b2的抑制作用。还评估了化合物的联合抑制作用,并使用Chou-Talalay方法处理了数据。通过平衡透析评估血浆蛋白结合。总共对沉麦中的49种皂苷进行了表征,并将其分为:10-100μmol/天(来自ShenMai的化合物剂量; 7种化合物),1-10μmol/天(17种化合物)和<1μmol/天(25种化合物,包括麦冬麦草碱)。给药后,循环的皂苷为原人参二醇型人参皂苷Rb1,Rb2,Rc,Rd,Ra1,Rg3,Ra2和Ra3,原人参三醇型人参皂苷Rg1,Re,Rg2和Rf以及人参皂苷Ro。原人参二醇型人参皂苷最大血浆浓度为2.1-46.6μmol/ L,血浆未结合分数为0.4-1.0%,终末半衰期为15.6-28.5 h(人参皂苷Rg3为1.9 h),而其他人参皂苷则为0.1-分别为7.7μmol/ L,20.8-99.2%和0.2-0.5 h。原人参二醇型人参皂甙,在C-20处没有任何糖附着的人参皂甙(人参皂甙Rf除外),人参皂甙Ro对OATP1B3的抑制作用(IC50,0.2-3.5 µmol / L)比其他人参皂甙(≥22.6µmol / L)更有力。人参皂甙对OATP1B1的抑制作用不如对OATP1B3的抑制作用强。人参皂苷Rb1,Rb2,Rc,Rd,Ro,Ra1,Re和Rg2可能以加成和时间相关的方式贡献了OATP1B3介导的深麦与药物相互作用的主要部分。









































 京公网安备 11010802027423号
京公网安备 11010802027423号