Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A protein functionalization platform based on selective reactions at methionine residues
Nature ( IF 50.5 ) Pub Date : 2018-10-01 , DOI: 10.1038/s41586-018-0608-y Michael T Taylor 1 , Jennifer E Nelson 1 , Marcos G Suero 1 , Matthew J Gaunt 1
Nature ( IF 50.5 ) Pub Date : 2018-10-01 , DOI: 10.1038/s41586-018-0608-y Michael T Taylor 1 , Jennifer E Nelson 1 , Marcos G Suero 1 , Matthew J Gaunt 1
Affiliation
|
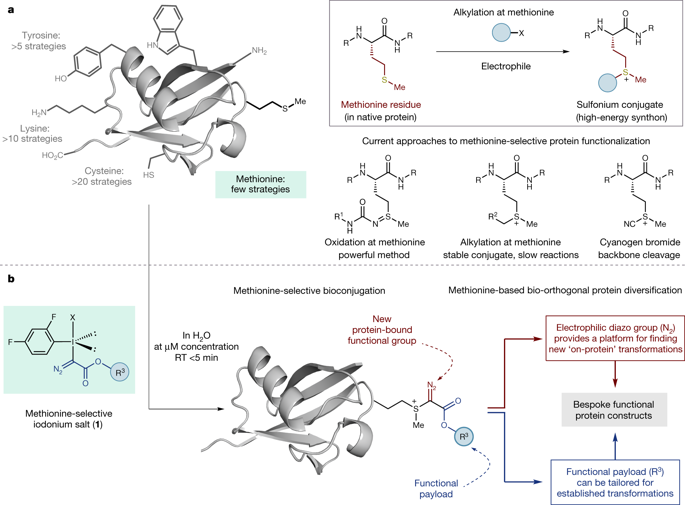
Nature has a remarkable ability to carry out site-selective post-translational modification of proteins, therefore enabling a marked increase in their functional diversity1. Inspired by this, chemical tools have been developed for the synthetic manipulation of protein structure and function, and have become essential to the continued advancement of chemical biology, molecular biology and medicine. However, the number of chemical transformations that are suitable for effective protein functionalization is limited, because the stringent demands inherent to biological systems preclude the applicability of many potential processes2. These chemical transformations often need to be selective at a single site on a protein, proceed with very fast reaction rates, operate under biologically ambient conditions and should provide homogeneous products with near-perfect conversion2–7. Although many bioconjugation methods exist at cysteine, lysine and tyrosine, a method targeting a less-explored amino acid would considerably expand the protein functionalization toolbox. Here we report the development of a multifaceted approach to protein functionalization based on chemoselective labelling at methionine residues. By exploiting the electrophilic reactivity of a bespoke hypervalent iodine reagent, the S-Me group in the side chain of methionine can be targeted. The bioconjugation reaction is fast, selective, operates at low-micromolar concentrations and is complementary to existing bioconjugation strategies. Moreover, it produces a protein conjugate that is itself a high-energy intermediate with reactive properties and can serve as a platform for the development of secondary, visible-light-mediated bioorthogonal protein functionalization processes. The merger of these approaches provides a versatile platform for the development of distinct transformations that deliver information-rich protein conjugates directly from the native biomacromolecules.This methionine-selective functionalization strategy uses hypervalent iodine reagents to introduce new groups via the formation of a sulfonium intermediate, which can then undergo further visible-light-mediated reactions to form a diverse range of protein conjugates.
中文翻译:

基于蛋氨酸残基选择性反应的蛋白质功能化平台
大自然具有对蛋白质进行位点选择性翻译后修饰的非凡能力,因此能够显着增加其功能多样性1。受此启发,化学工具被开发用于蛋白质结构和功能的合成操作,并且对于化学生物学、分子生物学和医学的持续发展至关重要。然而,适合有效蛋白质功能化的化学转化的数量是有限的,因为生物系统固有的严格要求排除了许多潜在过程的适用性2。这些化学转化通常需要在蛋白质的单个位点上具有选择性,以非常快的反应速率进行,在生物环境条件下进行,并且应该提供具有近乎完美转化的均质产物2-7。尽管针对半胱氨酸、赖氨酸和酪氨酸存在许多生物共轭方法,但针对较少探索的氨基酸的方法将大大扩展蛋白质功能化工具箱。在这里,我们报告了基于蛋氨酸残基化学选择性标记的多方面蛋白质功能化方法的发展。通过利用定制高价碘试剂的亲电子反应性,可以靶向甲硫氨酸侧链中的 S-Me 基团。生物共轭反应快速、选择性强,可在低微摩尔浓度下进行,并且是对现有生物共轭策略的补充。此外,它产生的蛋白质缀合物本身就是一种具有反应特性的高能中间体,可以作为开发二次可见光介导的生物正交蛋白质功能化过程的平台。这些方法的合并为开发不同的转化提供了一个通用平台,这些转化直接从天然生物大分子中提供信息丰富的蛋白质缀合物。这种蛋氨酸选择性功能化策略使用高价碘试剂通过形成锍中间体引入新基团,然后可以进行进一步的可见光介导的反应,形成多种蛋白质缀合物。
更新日期:2018-10-01
中文翻译:

基于蛋氨酸残基选择性反应的蛋白质功能化平台
大自然具有对蛋白质进行位点选择性翻译后修饰的非凡能力,因此能够显着增加其功能多样性1。受此启发,化学工具被开发用于蛋白质结构和功能的合成操作,并且对于化学生物学、分子生物学和医学的持续发展至关重要。然而,适合有效蛋白质功能化的化学转化的数量是有限的,因为生物系统固有的严格要求排除了许多潜在过程的适用性2。这些化学转化通常需要在蛋白质的单个位点上具有选择性,以非常快的反应速率进行,在生物环境条件下进行,并且应该提供具有近乎完美转化的均质产物2-7。尽管针对半胱氨酸、赖氨酸和酪氨酸存在许多生物共轭方法,但针对较少探索的氨基酸的方法将大大扩展蛋白质功能化工具箱。在这里,我们报告了基于蛋氨酸残基化学选择性标记的多方面蛋白质功能化方法的发展。通过利用定制高价碘试剂的亲电子反应性,可以靶向甲硫氨酸侧链中的 S-Me 基团。生物共轭反应快速、选择性强,可在低微摩尔浓度下进行,并且是对现有生物共轭策略的补充。此外,它产生的蛋白质缀合物本身就是一种具有反应特性的高能中间体,可以作为开发二次可见光介导的生物正交蛋白质功能化过程的平台。这些方法的合并为开发不同的转化提供了一个通用平台,这些转化直接从天然生物大分子中提供信息丰富的蛋白质缀合物。这种蛋氨酸选择性功能化策略使用高价碘试剂通过形成锍中间体引入新基团,然后可以进行进一步的可见光介导的反应,形成多种蛋白质缀合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号