当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gram‐Positive and Gram‐Negative Antibiotic Activity of Asymmetric and Monomeric Robenidine Analogues
ChemMedChem ( IF 3.6 ) Pub Date : 2018-11-19 , DOI: 10.1002/cmdc.201800463 Cecilia C. Russell 1 , Andrew Stevens 1 , Hongfei Pi 2 , Manouchehr Khazandi 2 , Abiodun D. Ogunniyi 2 , Kelly A. Young 1 , Jennifer R. Baker 1 , Siobhann N. McCluskey 1 , Stephen W. Page 3 , Darren J. Trott 2 , Adam McCluskey 1
ChemMedChem ( IF 3.6 ) Pub Date : 2018-11-19 , DOI: 10.1002/cmdc.201800463 Cecilia C. Russell 1 , Andrew Stevens 1 , Hongfei Pi 2 , Manouchehr Khazandi 2 , Abiodun D. Ogunniyi 2 , Kelly A. Young 1 , Jennifer R. Baker 1 , Siobhann N. McCluskey 1 , Stephen W. Page 3 , Darren J. Trott 2 , Adam McCluskey 1
Affiliation
|
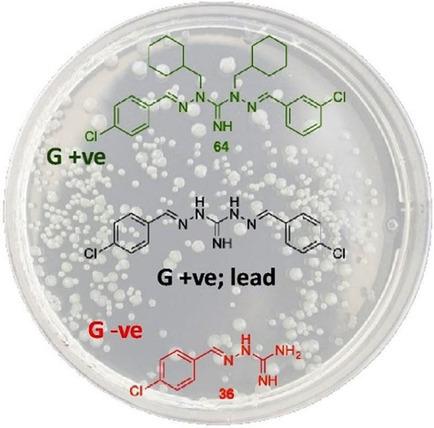
Desymmetrisation of robenidine (1: N′,2‐bis((E)‐4‐chlorobenzylidene)hydrazine‐1‐carboximidhydrazide) and the introduction of imine alkyl substituents gave good antibiotic activity. Of note was the increased potency of two analogues against vancomycin‐resistant Enterococci (VRE), one of which returned a MIC of 0.5 μg mL−1. Five analogues were found to be equipotent or more potent than the lead 1. Introduction of an indole moiety resulted in the most active robenidine analogue against methicillin‐resistant S. aureus (MRSA), with a MIC of 1.0 μg mL−1. Imine C=NH isosteres (C=O/C=S) were inactive. Monomeric analogues were 16–64 μg mL−1 active against MRSA and VRE. An analogue that lacks the terminal hydrazide NH moiety showed modest Gram‐negative activity at 64 μg mL−1. A 4‐tert‐butyl analogue was shown to be active against both Gram‐positive and ‐negative strains at 16–64 μg mL−1. In general, additional modifications with aromatic moieties was poorly tolerated, except with concomitant introduction of an imine C‐alkyl group. The activity of these analogues against MRSA and VRE ranged from 8 μg mL−1 to inactive (MIC>128 μg mL−1) with the naphthyl and indole analogues. Gram‐negative activity was most promising with two compounds at 16 μg mL−1 against E. coli. Against P. aeruginosa, the highest activity observed was with MIC values of 32 μg mL−1 with another two analogues. Combined, these findings support the further development of the (E)‐2‐benzylidenehydrazine‐1‐carboximidamide scaffold as a promising scaffold for the development of antibiotics against Gram‐positive and Gram‐negative strains.
中文翻译:

不对称和单体罗布定类似物的革兰氏阳性和革兰氏阴性抗生素活性
罗非替丁的去对称化(1:N ',2-二((E)-4-氯苄叉)肼-1-羧酰亚胺肼)和亚胺烷基取代基的引入具有良好的抗生素活性。值得注意的是,两种类似物对耐万古霉素的肠球菌(VRE)的效力有所提高,其中一种的MIC为0.5μgmL -1。发现有五个类似物比铅1具有更强的效价。吲哚部分的引入导致针对甲氧西林抗性金黄色葡萄球菌(MRSA)的活性最强的robenidine类似物,MIC为1.0μgmL -1。亚胺C = NH等位基因(C = O / C = S)不活跃。单体类似物为16–64μgmL-1对MRSA和VRE有效。缺少末端酰肼NH部分的类似物在64μgmL -1下显示适度的革兰氏阴性活性。研究表明,在16–64μgmL -1下, 4-叔丁基类似物对革兰氏阳性和阴性菌株均具有活性。通常,除伴随引入亚胺C-烷基外,对芳香族基团的其他修饰耐受性差。这些类似物对MRSA和VRE的活性范围为8μgmL -1到萘基和吲哚类似物无活性(MIC> 128μgmL -1)。用两种浓度为16μgmL -1的化合物对大肠杆菌的革兰氏阴性活性最有前途。反对铜绿假单胞菌,观察到的最高活性是与另外两个类似物的MIC值为32μgmL -1。综合起来,这些发现支持(E)-2-苄叉肼-1-羧酰亚胺酰胺支架的进一步开发,作为开发针对革兰氏阳性和革兰氏阴性菌株的抗生素的有前途的支架。
更新日期:2018-11-19
中文翻译:

不对称和单体罗布定类似物的革兰氏阳性和革兰氏阴性抗生素活性
罗非替丁的去对称化(1:N ',2-二((E)-4-氯苄叉)肼-1-羧酰亚胺肼)和亚胺烷基取代基的引入具有良好的抗生素活性。值得注意的是,两种类似物对耐万古霉素的肠球菌(VRE)的效力有所提高,其中一种的MIC为0.5μgmL -1。发现有五个类似物比铅1具有更强的效价。吲哚部分的引入导致针对甲氧西林抗性金黄色葡萄球菌(MRSA)的活性最强的robenidine类似物,MIC为1.0μgmL -1。亚胺C = NH等位基因(C = O / C = S)不活跃。单体类似物为16–64μgmL-1对MRSA和VRE有效。缺少末端酰肼NH部分的类似物在64μgmL -1下显示适度的革兰氏阴性活性。研究表明,在16–64μgmL -1下, 4-叔丁基类似物对革兰氏阳性和阴性菌株均具有活性。通常,除伴随引入亚胺C-烷基外,对芳香族基团的其他修饰耐受性差。这些类似物对MRSA和VRE的活性范围为8μgmL -1到萘基和吲哚类似物无活性(MIC> 128μgmL -1)。用两种浓度为16μgmL -1的化合物对大肠杆菌的革兰氏阴性活性最有前途。反对铜绿假单胞菌,观察到的最高活性是与另外两个类似物的MIC值为32μgmL -1。综合起来,这些发现支持(E)-2-苄叉肼-1-羧酰亚胺酰胺支架的进一步开发,作为开发针对革兰氏阳性和革兰氏阴性菌株的抗生素的有前途的支架。











































 京公网安备 11010802027423号
京公网安备 11010802027423号