International Journal of Hydrogen Energy ( IF 8.1 ) Pub Date : 2018-10-12 , DOI: 10.1016/j.ijhydene.2018.09.121 P.L. Rodríguez-Kessler , Fernando Murillo , A.R. Rodríguez-Domínguez , Pedro Navarro-Santos , Gabriel Merino
|
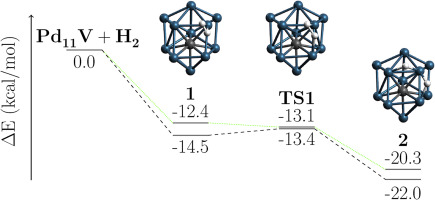
The structure and stability of V-doped Pdn (n = 2–12) clusters as well as their ability for hydrogen dissociation are analyzed using a successive growth algorithm coupled with density functional theory (DFT) computations. From the structural point of view, the lowest energy structures of these clusters are three-dimensional with exohedral geometries for n = 2–7 whereas endohedral for n = 8 onward. From their second-order energy differences, Pd4V and Pd10V are found to be the most stable ones. Among the PdnV(H2) complexes, Pd6V2H possesses the highest stability, as it is supported by the chemisorption energy, the vertical ionization potential (VIP), and the vertical electron affinity (VEA), respectively. Most importantly, the hydrogen dissociation pathway on PdnV clusters with n = 3, 4 and 10–12 shows that these clusters are rigid and suitable to dissociate H2 while for n = 5–9 the structure of the clusters changes. The H2 dissociation process on PdnV clusters with n = 8, 10, and 11 carries out barrierless.
中文翻译:

V掺杂的Pd n(n = 2–12)团簇的结构及其对H 2的解离能力
使用连续生长算法结合密度泛函理论(DFT)计算,分析了V掺杂的Pd n(n = 2-12)团簇的结构和稳定性,以及它们的氢解离能力。从结构的角度来看,这些团簇的最低能级结构是三维的,其中n = 2–7的面外几何形状,而n = 8以后的内面几何形状。根据它们的二阶能量差,发现Pd 4 V和Pd 10 V是最稳定的。在Pd n V(H 2)配合物中,Pd 6 V2H具有最高的稳定性,因为它分别受化学吸附能,垂直电离能(VIP)和垂直电子亲和力(VEA)的支持。最重要的是,n = 3、4和10–12的Pd n V团簇上的氢离解途径表明,这些团簇是刚性的,适合解离H 2,而对于n = 5–9,团簇的结构却发生了变化。在n = 8、10和11的Pd n V团簇上的H 2离解过程可以无障碍地进行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号