当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic enantioselective ene-type reactions of vinylogous hydrazone: construction of α-methylene-γ-butyrolactone derivatives†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-10-13 00:00:00 , DOI: 10.1039/c8cc07567k Hang Zhang 1, 2, 3, 4, 5 , Qian Yao 1, 2, 3, 4, 5 , Weidi Cao 1, 2, 3, 4, 5 , Shulin Ge 1, 2, 3, 4, 5 , Jinxiu Xu 1, 2, 3, 4, 5 , Xiaohua Liu 1, 2, 3, 4, 5 , Xiaoming Feng 1, 2, 3, 4, 5
Chemical Communications ( IF 4.3 ) Pub Date : 2018-10-13 00:00:00 , DOI: 10.1039/c8cc07567k Hang Zhang 1, 2, 3, 4, 5 , Qian Yao 1, 2, 3, 4, 5 , Weidi Cao 1, 2, 3, 4, 5 , Shulin Ge 1, 2, 3, 4, 5 , Jinxiu Xu 1, 2, 3, 4, 5 , Xiaohua Liu 1, 2, 3, 4, 5 , Xiaoming Feng 1, 2, 3, 4, 5
Affiliation
|
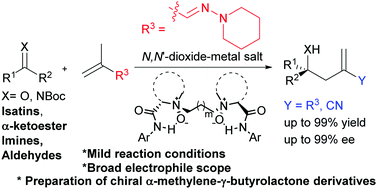
The catalytic asymmetric ene-type reactions of vinylogous hydrazone were accomplished by using chiral N,N′-dioxide–metal salt complexes as catalysts. A wide range of electrophiles, including isatins, α-ketoester, imines, and aldehydes reacted with (E)-2-methyl-N-(piperidin-1-yl)prop-2-en-1-imine efficiently, affording the corresponding homoallylic alcohols and amines in high yields (up to 99%) with excellent ee values (up to 99%). The methodology provided a convenient way to synthesize bioactive chiral α-methylene-γ-butyrolactone derivatives.
中文翻译:

乙烯基的催化对映选择性烯类反应:α-亚甲基-γ-丁内酯衍生物的构建†
乙烯基ous的催化不对称烯型反应是通过使用手性N,N'-二氧化物-金属盐络合物作为催化剂来完成的。各种各样的亲电子试剂,包括靛红,α-酮酸酯,亚胺和醛,可以与(E)-2-甲基-N-(哌啶-1-基)丙-2-烯-1-亚胺有效地反应,得到相应的均丙醇和胺,收率高(高达99%),具有出色的ee值(高达99%)。该方法提供了合成生物活性手性α-亚甲基-γ-丁内酯衍生物的简便方法。
更新日期:2018-10-13
中文翻译:

乙烯基的催化对映选择性烯类反应:α-亚甲基-γ-丁内酯衍生物的构建†
乙烯基ous的催化不对称烯型反应是通过使用手性N,N'-二氧化物-金属盐络合物作为催化剂来完成的。各种各样的亲电子试剂,包括靛红,α-酮酸酯,亚胺和醛,可以与(E)-2-甲基-N-(哌啶-1-基)丙-2-烯-1-亚胺有效地反应,得到相应的均丙醇和胺,收率高(高达99%),具有出色的ee值(高达99%)。该方法提供了合成生物活性手性α-亚甲基-γ-丁内酯衍生物的简便方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号