当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic properties of MgGa2O4 and phase relations in the system Mg-Ga-O
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.jallcom.2018.10.147 K.T. Jacob , Shivesh Sivakumar
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2019-02-01 , DOI: 10.1016/j.jallcom.2018.10.147 K.T. Jacob , Shivesh Sivakumar
|
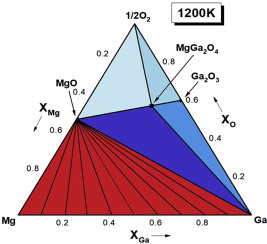
Abstract Materials based on MgGa2O4 find use in microwave dielectrics, optoelectronics, spintronics and solid electrolytes for high-temperature fuel cells. For designing optimum processing conditions and evaluating interactions with other materials and environments, thermodynamic data and appropriate phase diagrams are required. As part of a larger systematic study on spinels with functional applications, thermodynamic properties of MgGa2O4 are determined in the temperature range from 875 to 1325 K using an electrochemical cell incorporating yttria-stabilized zirconia as the solid electrolyte. The Gibbs energy of formation of MgGa2O4 from its constituent binary oxides MgO with halite structure and β-Ga2O3 with monoclinic structure is obtained: Δ G f ( ox ) o (±135)/J mol−1 = –39868–8.742 (T/K). The heat capacity of MgGa2O4 in the temperature range from 310 to 1200 K is measured by DSC. Both positive and negative deviations from Neumann-Kopp rule are seen in different temperature ranges. Derived from these measurements are the standard enthalpy of formation and standard entropy of MgGa2O4 at 298.15 K. The isothermal section of the phase diagram of the system Mg-Ga-O and oxygen potential-composition diagram at 1200 K are computed from thermodynamic data.
中文翻译:

MgGa2O4 的热力学性质和 Mg-Ga-O 体系中的相关系
摘要 基于 MgGa2O4 的材料可用于微波电介质、光电子学、自旋电子学和高温燃料电池的固体电解质。为了设计最佳加工条件并评估与其他材料和环境的相互作用,需要热力学数据和适当的相图。作为对具有功能应用的尖晶石的更大系统研究的一部分,MgGa2O4 的热力学性质是在 875 到 1325 K 的温度范围内使用包含氧化钇稳定的氧化锆作为固体电解质的电化学电池确定的。由其组成的具有岩盐结构的二元氧化物 MgO 和具有单斜结构的 β-Ga2O3 生成 MgGa2O4 的吉布斯能是: Δ G f ( ox ) o (±135)/J mol−1 = –39868–8.742 (T/ K)。MgGa2O4 在 310 到 1200 K 温度范围内的热容量是通过 DSC 测量的。在不同的温度范围内都可以看到与 Neumann-Kopp 规则的正偏差和负偏差。从这些测量中推导出 MgGa2O4 在 298.15 K 时的标准生成焓和标准熵。系统 Mg-Ga-O 相图的等温截面和 1200 K 时的氧势-组成图是根据热力学数据计算的。
更新日期:2019-02-01
中文翻译:

MgGa2O4 的热力学性质和 Mg-Ga-O 体系中的相关系
摘要 基于 MgGa2O4 的材料可用于微波电介质、光电子学、自旋电子学和高温燃料电池的固体电解质。为了设计最佳加工条件并评估与其他材料和环境的相互作用,需要热力学数据和适当的相图。作为对具有功能应用的尖晶石的更大系统研究的一部分,MgGa2O4 的热力学性质是在 875 到 1325 K 的温度范围内使用包含氧化钇稳定的氧化锆作为固体电解质的电化学电池确定的。由其组成的具有岩盐结构的二元氧化物 MgO 和具有单斜结构的 β-Ga2O3 生成 MgGa2O4 的吉布斯能是: Δ G f ( ox ) o (±135)/J mol−1 = –39868–8.742 (T/ K)。MgGa2O4 在 310 到 1200 K 温度范围内的热容量是通过 DSC 测量的。在不同的温度范围内都可以看到与 Neumann-Kopp 规则的正偏差和负偏差。从这些测量中推导出 MgGa2O4 在 298.15 K 时的标准生成焓和标准熵。系统 Mg-Ga-O 相图的等温截面和 1200 K 时的氧势-组成图是根据热力学数据计算的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号