Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Functional Carbo-benzenes with Functional Properties: The C2 Tether Key
Synlett ( IF 1.7 ) Pub Date : 2018-10-12 , DOI: 10.1055/s-0037-1610269 Valérie Maraval 1, 2 , Remi Chauvin 1, 2 , Kévin Cocq 1, 2 , Cécile Barthes 1, 2 , Arnaud Rives 1, 2
Synlett ( IF 1.7 ) Pub Date : 2018-10-12 , DOI: 10.1055/s-0037-1610269 Valérie Maraval 1, 2 , Remi Chauvin 1, 2 , Kévin Cocq 1, 2 , Cécile Barthes 1, 2 , Arnaud Rives 1, 2
Affiliation
|
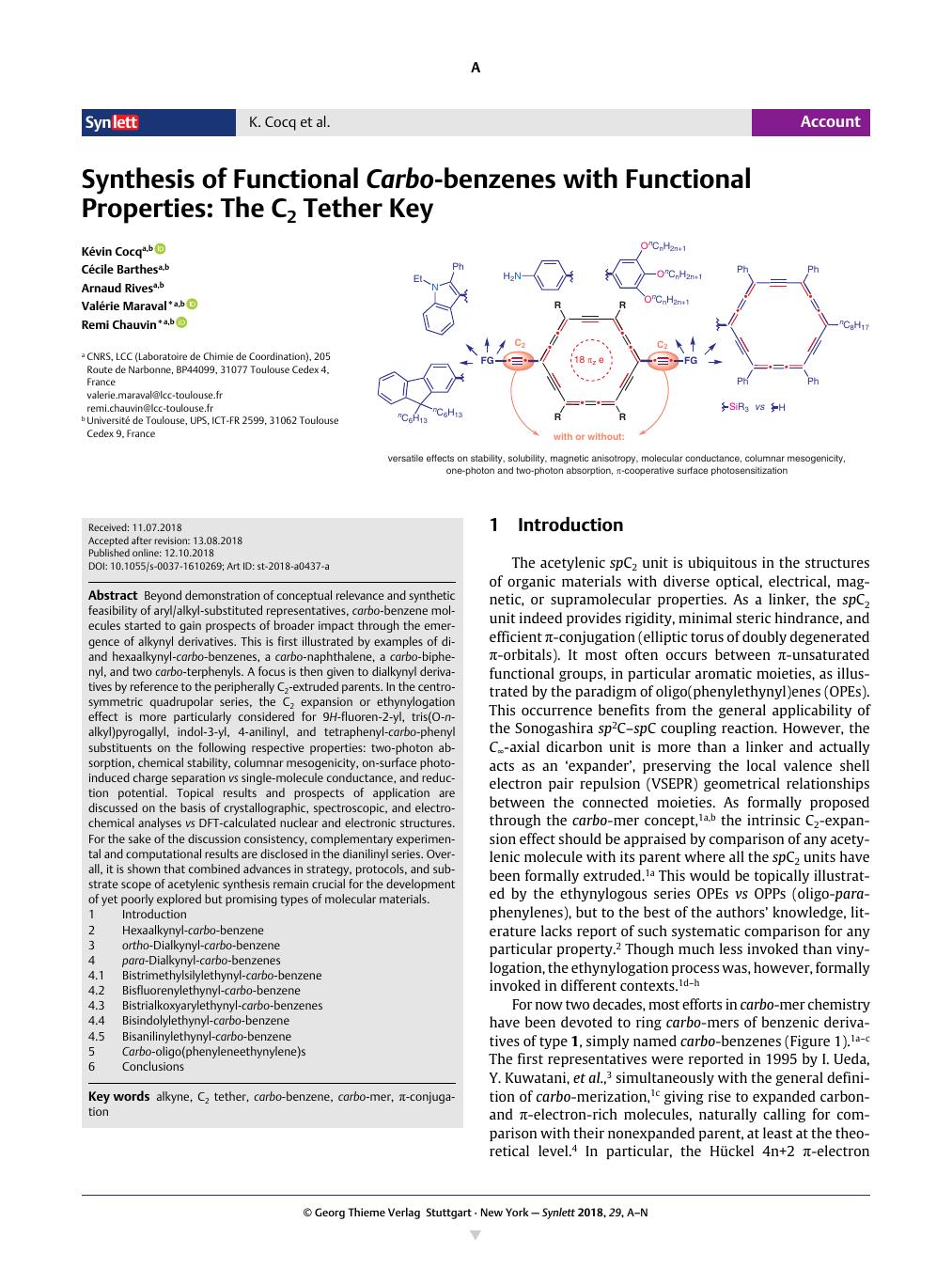
Beyond demonstration of conceptual relevance and synthetic feasibility of aryl/alkyl-substituted representatives, carbo-benzene molecules started to gain prospects of broader impact through the emergence of alkynyl derivatives. This is first illustrated by examples of di- and hexaalkynyl-carbo-benzenes, a carbo-naphthalene, a carbo-biphenyl, and two carbo-terphenyls. A focus is then given to dialkynyl derivatives by reference to the peripherally C2-extruded parents. In the centrosymmetric quadrupolar series, the C2 expansion or ethynylogation effect is more particularly considered for 9H-fluoren-2-yl, tris(O-n-alkyl)pyrogallyl, indol-3-yl, 4-anilinyl, and tetraphenyl-carbo-phenyl substituents on the following respective properties: two-photon absorption, chemical stability, columnar mesogenicity, on-surface photoinduced charge separation vs single-molecule conductance, and reduction potential. Topical results and prospects of application are discussed on the basis of crystallographic, spectroscopic, and electrochemical analyses vs DFT-calculated nuclear and electronic structures. For the sake of the discussion consistency, complementary experimental and computational results are disclosed in the dianilinyl series. Overall, it is shown that combined advances in strategy, protocols, and substrate scope of acetylenic synthesis remain crucial for the development of yet poorly explored but promising types of molecular materials. 1 Introduction 2 Hexaalkynyl-carbo-benzene 3 ortho-Dialkynyl-carbo-benzene 4 para-Dialkynyl-carbo-benzenes 4.1 Bistrimethylsilylethynyl-carbo-benzene 4.2 Bisfluorenylethynyl-carbo-benzene 4.3 Bistrialkoxyarylethynyl-carbo-benzenes 4.4 Bisindolylethynyl-carbo-benzene 4.5 Bisanilinylethynyl-carbo-benzene 5 Carbo-oligo(phenyleneethynylene)s 6 Conclusions
中文翻译:

具有功能特性的功能性碳苯的合成:C2 Tether Key
除了证明芳基/烷基取代代表的概念相关性和合成可行性之外,通过炔基衍生物的出现,碳苯分子开始获得更广泛影响的前景。这首先通过二-和六炔基-碳-苯、一个碳-萘、一个碳-联苯和两个碳-三联苯的例子来说明。然后通过参考外围 C2 挤出的母体将重点放在二炔基衍生物上。在中心对称四极系列中,对于 9H-芴-2-基、三(On-烷基)焦烯丙基、吲哚-3-基、4-苯胺基和四苯基-碳-苯基取代基,更特别考虑了 C2 扩展或乙炔化效应关于以下各自的特性:双光子吸收、化学稳定性、柱状介晶性、表面光致电荷分离与单分子电导和还原电位。在晶体学、光谱学和电化学分析与 DFT 计算的核和电子结构的基础上讨论了局部结果和应用前景。为了讨论的一致性,在二苯胺系列中公开了补充实验和计算结果。总体而言,结果表明,炔合成的策略、方案和底物范围的综合进步对于开发尚未充分探索但有前途的分子材料类型仍然至关重要。1 引言 2 六炔基碳苯 3 邻二炔基碳苯 4 对二炔基碳苯 4.1 双三甲基甲硅烷基乙炔基碳苯 4.2 双芴乙炔基碳苯 4.
更新日期:2018-10-12
中文翻译:

具有功能特性的功能性碳苯的合成:C2 Tether Key
除了证明芳基/烷基取代代表的概念相关性和合成可行性之外,通过炔基衍生物的出现,碳苯分子开始获得更广泛影响的前景。这首先通过二-和六炔基-碳-苯、一个碳-萘、一个碳-联苯和两个碳-三联苯的例子来说明。然后通过参考外围 C2 挤出的母体将重点放在二炔基衍生物上。在中心对称四极系列中,对于 9H-芴-2-基、三(On-烷基)焦烯丙基、吲哚-3-基、4-苯胺基和四苯基-碳-苯基取代基,更特别考虑了 C2 扩展或乙炔化效应关于以下各自的特性:双光子吸收、化学稳定性、柱状介晶性、表面光致电荷分离与单分子电导和还原电位。在晶体学、光谱学和电化学分析与 DFT 计算的核和电子结构的基础上讨论了局部结果和应用前景。为了讨论的一致性,在二苯胺系列中公开了补充实验和计算结果。总体而言,结果表明,炔合成的策略、方案和底物范围的综合进步对于开发尚未充分探索但有前途的分子材料类型仍然至关重要。1 引言 2 六炔基碳苯 3 邻二炔基碳苯 4 对二炔基碳苯 4.1 双三甲基甲硅烷基乙炔基碳苯 4.2 双芴乙炔基碳苯 4.











































 京公网安备 11010802027423号
京公网安备 11010802027423号