当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Replacement of the Benzylpiperidine Moiety with Fluorinated Phenylalkyl Side Chains for the Development of GluN2B Receptor Ligands
ChemMedChem ( IF 3.4 ) Pub Date : 2018-11-14 , DOI: 10.1002/cmdc.201800566 Simone Thum 1 , Dirk Schepmann 1 , Dmitrii V. Kalinin 1 , Simon M. Ametamey 2 , Bernhard Wünsch 1, 3
ChemMedChem ( IF 3.4 ) Pub Date : 2018-11-14 , DOI: 10.1002/cmdc.201800566 Simone Thum 1 , Dirk Schepmann 1 , Dmitrii V. Kalinin 1 , Simon M. Ametamey 2 , Bernhard Wünsch 1, 3
Affiliation
|
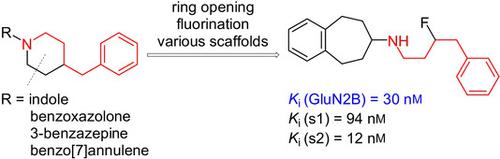
The 4‐benzylpiperidine moiety is a central structural element of potent N‐methyl‐d‐aspartate (NMDA) receptor antagonists containing the GluN2B subunit. To obtain novel GluN2B ligands suitable for positron emission tomography, the benzylpiperidine moiety was replaced with fluorinated ω‐phenylalkylamino groups. For this purpose three primary propyl‐ and butylamines 7 a–c and one butyraldehyde 7 d bearing a fluorine atom and an ω‐phenyl moiety were prepared in 3‐ to 7‐step syntheses. Compounds 7 a–d were attached to various scaffolds of potent GluN2B antagonists (scaffold hopping) instead of the original 4‐benzylpiperidine moiety. Although benzoxazol‐2‐ones and indoles with a benzylpiperidine moiety show high GluN2B affinity, the corresponding fluorophenylalkylamine derivatives did not result in high Glu2B affinity. Moderate GluN2B affinity was observed for a 3‐(fluoroalkyl)‐substituted tetrahydro‐1H‐3‐benzazepine (Ki=239 nm). However, high GluN2B affinity was obtained for the tetrahydro‐5H‐benzo[7]annulen‐7‐amines 12 a–c (Ki=17–30 nm). Docking studies resulted in the same binding pose for 12 a as for the lead compound Ro 25‐6981. It can be concluded that some GluN2B ligands (benzoxazolones, indoles) do not tolerate replacement of the 4‐benzylpiperidine moiety with flexible fluorinated phenylalkyl side chains, but other scaffolds such as tetrahydro‐3‐benzazepines and ‐benzo[7]annulenes retain interaction with NMDA receptors containing the GluN2B subunit.
中文翻译:

用氟化的苯基烷基侧链取代苄基哌啶部分用于开发GluN2B受体配体
4-苄基哌啶基部分是强效的中央结构元件Ñ甲基d含有GluN2B亚基天冬氨酸(NMDA)受体拮抗剂。为了获得适用于正电子发射断层扫描的新型GluN2B配体,苄基哌啶部分被氟化的ω-苯基烷基氨基取代。为此,通过3到7步合成过程制备了三个带有氟原子和ω-苯基部分的伯丙胺和丁胺7a - c和一个丁醛7d。化合物7 a – d被连接到各种有效的GluN2B拮抗剂支架上(支架跳跃),而不是最初的4-苄基哌啶部分。尽管具有苄基哌啶部分的苯并恶唑-2-酮和吲哚显示出较高的GluN2B亲和力,但相应的氟苯基烷基胺衍生物并未产生较高的Glu2B亲和力。对于3-(氟代烷基)取代的四氢-1 H -3苯并ze庚因(K i = 239 n m),观察到中等的GluN2B亲和力。但是,对四氢-5 H苯并[7]年戊烯-7胺12 a – c(K i = 17–30 n m)获得了很高的GluN2B亲和力。对接研究导致相同的约束姿势铅化合物Ro 25‐6981为12 a。可以得出结论,某些GluN2B配体(苯并恶唑酮,吲哚类)不容许用柔性的氟化苯基烷基侧链取代4-苄基哌啶部分,但其他支架如四氢-3-苯并enza庚因和苯并[7]环丁烯则保留与含有GluN2B亚基的NMDA受体。
更新日期:2018-11-14
中文翻译:

用氟化的苯基烷基侧链取代苄基哌啶部分用于开发GluN2B受体配体
4-苄基哌啶基部分是强效的中央结构元件Ñ甲基d含有GluN2B亚基天冬氨酸(NMDA)受体拮抗剂。为了获得适用于正电子发射断层扫描的新型GluN2B配体,苄基哌啶部分被氟化的ω-苯基烷基氨基取代。为此,通过3到7步合成过程制备了三个带有氟原子和ω-苯基部分的伯丙胺和丁胺7a - c和一个丁醛7d。化合物7 a – d被连接到各种有效的GluN2B拮抗剂支架上(支架跳跃),而不是最初的4-苄基哌啶部分。尽管具有苄基哌啶部分的苯并恶唑-2-酮和吲哚显示出较高的GluN2B亲和力,但相应的氟苯基烷基胺衍生物并未产生较高的Glu2B亲和力。对于3-(氟代烷基)取代的四氢-1 H -3苯并ze庚因(K i = 239 n m),观察到中等的GluN2B亲和力。但是,对四氢-5 H苯并[7]年戊烯-7胺12 a – c(K i = 17–30 n m)获得了很高的GluN2B亲和力。对接研究导致相同的约束姿势铅化合物Ro 25‐6981为12 a。可以得出结论,某些GluN2B配体(苯并恶唑酮,吲哚类)不容许用柔性的氟化苯基烷基侧链取代4-苄基哌啶部分,但其他支架如四氢-3-苯并enza庚因和苯并[7]环丁烯则保留与含有GluN2B亚基的NMDA受体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号