当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NO2 Suppression of Autoxidation-Inhibition of Gas-Phase Highly Oxidized Dimer Product Formation.
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-10-12 , DOI: 10.1021/acsearthspacechem.8b00123 Matti P Rissanen 1
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-10-12 , DOI: 10.1021/acsearthspacechem.8b00123 Matti P Rissanen 1
Affiliation
|
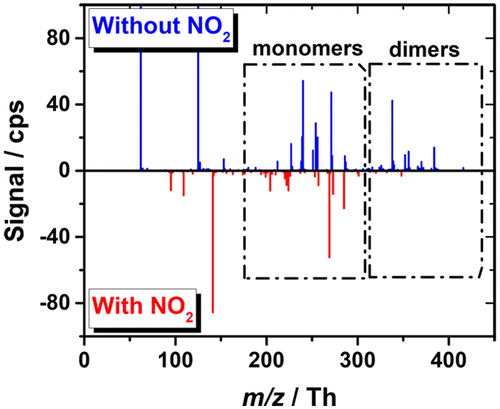
Atmospheric autoxidation of volatile organic compounds (VOC) leads to prompt formation of highly oxidized multifunctional compounds (HOM) that have been found crucial in forming ambient secondary organic aerosol (SOA). As a radical chain reaction mediated by oxidized peroxy (RO2) and alkoxy (RO) radical intermediates, the formation pathways can be intercepted by suitable reaction partners, preventing the production of the highest oxidized reaction products, and thus the formation of the most condensable material. Commonly, NO is expected to have a detrimental effect on RO2 chemistry, and thus on autoxidation, whereas the influence of NO2 is mostly neglected. Here it is shown by dedicated flow tube experiments, how high concentration of NO2 suppresses cyclohexene ozonolysis initiated autoxidation chain reaction. Importantly, the addition of NO2 ceases covalently bound dimer production, indicating their production involving acylperoxy radical (RC(O)OO•) intermediates. In related experiments NO was also shown to strongly suppress the highly oxidized product formation, but due to possibility for chain propagating reactions (as with RO2 and HO2 too), the suppression is not as absolute as with NO2. Furthermore, it is shown how NO x reactions with oxidized peroxy radicals lead into indistinguishable product compositions, complicating mass spectral assignments in any RO2 + NO x system. The present work was conducted with atmospheric pressure chemical ionization mass spectrometry (CIMS) as the detection method for the highly oxidized end-products and peroxy radical intermediates, under ambient conditions and at short few second reaction times. Specifically, the insight was gained by addition of a large amount of NO2 (and NO) to the oxidation system, upon which acylperoxy radicals reacted in RC(O)O2 + NO2 → RC(O)O2NO2 reaction to form peroxyacylnitrates, consequently shutting down the oxidation sequence.
中文翻译:

NO2 抑制自氧化-抑制气相高度氧化二聚体产物的形成。
挥发性有机化合物 (VOC) 的大气自氧化会导致高度氧化的多功能化合物 (HOM) 迅速形成,这些化合物被发现对于形成环境二次有机气溶胶 (SOA) 至关重要。作为由氧化过氧(RO2)和烷氧基(RO)自由基中间体介导的自由基链式反应,形成途径可以被合适的反应伙伴拦截,防止最高氧化反应产物的产生,从而形成最可凝结的材料。通常,NO 预计会对 RO2 化学反应产生不利影响,从而对自氧化产生不利影响,而 NO2 的影响大多被忽略。这里通过专用流管实验展示了高浓度的NO2如何抑制环己烯臭氧分解引发的自氧化链式反应。重要的是,添加NO2 会停止共价结合二聚体的产生,表明它们的产生涉及酰基过氧自由基(RC(O)OO•) 中间体。在相关实验中,NO 也被证明可以强烈抑制高度氧化产物的形成,但由于可能发生链增长反应(与 RO2 和 HO2 一样),其抑制作用并不像 NO2 那样绝对。此外,还显示了 NO x 与氧化过氧自由基的反应如何导致无法区分的产物成分,从而使任何 RO2 + NO x 系统中的质谱分配变得复杂。目前的工作是在环境条件下和短短几秒的反应时间内,使用常压化学电离质谱(CIMS)作为高度氧化的最终产物和过氧自由基中间体的检测方法进行的。具体来说,通过向氧化系统中添加大量NO2(和NO),酰基过氧自由基发生RC(O)O2 + NO2 → RC(O)O2NO2反应,形成过氧酰基硝酸盐,从而导致停工氧化顺序。
更新日期:2018-10-12
中文翻译:

NO2 抑制自氧化-抑制气相高度氧化二聚体产物的形成。
挥发性有机化合物 (VOC) 的大气自氧化会导致高度氧化的多功能化合物 (HOM) 迅速形成,这些化合物被发现对于形成环境二次有机气溶胶 (SOA) 至关重要。作为由氧化过氧(RO2)和烷氧基(RO)自由基中间体介导的自由基链式反应,形成途径可以被合适的反应伙伴拦截,防止最高氧化反应产物的产生,从而形成最可凝结的材料。通常,NO 预计会对 RO2 化学反应产生不利影响,从而对自氧化产生不利影响,而 NO2 的影响大多被忽略。这里通过专用流管实验展示了高浓度的NO2如何抑制环己烯臭氧分解引发的自氧化链式反应。重要的是,添加NO2 会停止共价结合二聚体的产生,表明它们的产生涉及酰基过氧自由基(RC(O)OO•) 中间体。在相关实验中,NO 也被证明可以强烈抑制高度氧化产物的形成,但由于可能发生链增长反应(与 RO2 和 HO2 一样),其抑制作用并不像 NO2 那样绝对。此外,还显示了 NO x 与氧化过氧自由基的反应如何导致无法区分的产物成分,从而使任何 RO2 + NO x 系统中的质谱分配变得复杂。目前的工作是在环境条件下和短短几秒的反应时间内,使用常压化学电离质谱(CIMS)作为高度氧化的最终产物和过氧自由基中间体的检测方法进行的。具体来说,通过向氧化系统中添加大量NO2(和NO),酰基过氧自由基发生RC(O)O2 + NO2 → RC(O)O2NO2反应,形成过氧酰基硝酸盐,从而导致停工氧化顺序。










































 京公网安备 11010802027423号
京公网安备 11010802027423号