当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Co-assembly of sugar-based amphiphilic block polymers to achieve nanoparticles with tunable morphology, size, surface charge, and acid-responsive behavior†
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2018-10-11 00:00:00 , DOI: 10.1039/c8qm00353j Yen-Nan Lin 1, 2, 3, 4, 5 , Lu Su 1, 2, 3, 4 , Justin Smolen 1, 2, 3, 4 , Richen Li 1, 2, 3, 4 , Yue Song 1, 2, 3, 4 , Hai Wang 1, 2, 3, 4 , Mei Dong 1, 2, 3, 4 , Karen L. Wooley 1, 2, 3, 4
Materials Chemistry Frontiers ( IF 7 ) Pub Date : 2018-10-11 00:00:00 , DOI: 10.1039/c8qm00353j Yen-Nan Lin 1, 2, 3, 4, 5 , Lu Su 1, 2, 3, 4 , Justin Smolen 1, 2, 3, 4 , Richen Li 1, 2, 3, 4 , Yue Song 1, 2, 3, 4 , Hai Wang 1, 2, 3, 4 , Mei Dong 1, 2, 3, 4 , Karen L. Wooley 1, 2, 3, 4
Affiliation
|
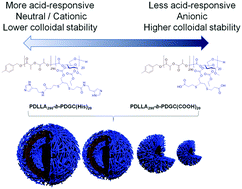
The development of next-generation smart nanocarriers that can be tailored for specific applications requires precise control over physiochemical properties, yet modulation of nanostructures solely through synthetic routes is a time-consuming and labor-intensive process. In this work, co-assembly of two degradable glucose-based amphiphilic block polymers is demonstrated as a means to control nanoparticle size, surface charge, and stimuli-responsive properties, allowing optimization of these constructs for cytosolic drug delivery applications. Polymeric particles with varying weight fractions of carboxylate- and histamine-modified poly(DL-lactide)-b-poly(D-glucose carbonate)s (PDLLA-b-PDGC) were obtained with diameters ranging from ca. 30 nm to 3 μm and zeta potential values ranging from ca. −35 mV to −1.6 mV in nanopure water. Histamine moieties imparted pH-responsive behavior due to protonation below pH 7, whereas the carboxylates imparted colloidal stability and anionic character. Blending the acid- and histamine-functionalized polymers produced co-assemblies with different pH-dependent surface charge profiles. In particular, co-assemblies with 60 wt% histamine-modified PDLLA-b-PDGC (fhistamine = 0.6) swelled upon acidification from physiological pH (7.4) to endolysosomal pH (5.5), which is anticipated to enable drug release within endolysosomal compartments. The accessible procedures presented here for engineering highly tunable nanoparticles from glucose-based, functional, degradable polymers offer versatile strategies for accelerating the development and clinical implementation of such stimuli-responsive, tailored nanocarriers.
中文翻译:

糖基两亲嵌段聚合物的共组装,以实现具有可调整的形态,尺寸,表面电荷和酸响应行为的纳米粒子†
可以为特定应用量身定制的下一代智能纳米载体的开发需要对物理化学性质进行精确控制,但是仅通过合成途径对纳米结构进行调节是一项耗时且劳动密集的过程。在这项工作中,两种可降解的基于葡萄糖的两亲性嵌段聚合物的共组装被证明是一种控制纳米颗粒大小,表面电荷和刺激响应特性的方法,从而可以优化这些构建物的胞质药物递送应用。获得具有不同重量分数的羧酸盐和组胺改性的聚(DL-丙交酯)-b-聚(D-葡萄糖碳酸酯)(PDLLA- b- PDGC)的聚合物颗粒,其直径范围为约。30 nm至3μm,ζ电位值范围约为 纳米纯水中的-35 mV至-1.6 mV。组胺部分由于在pH 7以下质子化而赋予pH响应行为,而羧酸盐具有胶体稳定性和阴离子特性。共混酸和组胺官能化的聚合物可产生具有不同的pH依赖性表面电荷分布的共组装体。特别是与60 wt%组胺修饰的PDLLA- b -PDGC(f组胺= 0.6)在酸化后从生理pH值(7.4)溶胀至溶酶体pH(5.5)膨胀,这有望使药物在溶酶体区室中释放。本文介绍的用于从葡萄糖基,功能性,可降解聚合物工程化高度可调纳米粒子的可访问程序为加速此类刺激响应性,量身定制的纳米载体的开发和临床实施提供了多种策略。
更新日期:2018-10-11
中文翻译:

糖基两亲嵌段聚合物的共组装,以实现具有可调整的形态,尺寸,表面电荷和酸响应行为的纳米粒子†
可以为特定应用量身定制的下一代智能纳米载体的开发需要对物理化学性质进行精确控制,但是仅通过合成途径对纳米结构进行调节是一项耗时且劳动密集的过程。在这项工作中,两种可降解的基于葡萄糖的两亲性嵌段聚合物的共组装被证明是一种控制纳米颗粒大小,表面电荷和刺激响应特性的方法,从而可以优化这些构建物的胞质药物递送应用。获得具有不同重量分数的羧酸盐和组胺改性的聚(DL-丙交酯)-b-聚(D-葡萄糖碳酸酯)(PDLLA- b- PDGC)的聚合物颗粒,其直径范围为约。30 nm至3μm,ζ电位值范围约为 纳米纯水中的-35 mV至-1.6 mV。组胺部分由于在pH 7以下质子化而赋予pH响应行为,而羧酸盐具有胶体稳定性和阴离子特性。共混酸和组胺官能化的聚合物可产生具有不同的pH依赖性表面电荷分布的共组装体。特别是与60 wt%组胺修饰的PDLLA- b -PDGC(f组胺= 0.6)在酸化后从生理pH值(7.4)溶胀至溶酶体pH(5.5)膨胀,这有望使药物在溶酶体区室中释放。本文介绍的用于从葡萄糖基,功能性,可降解聚合物工程化高度可调纳米粒子的可访问程序为加速此类刺激响应性,量身定制的纳米载体的开发和临床实施提供了多种策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号