当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Trajectory and Cycle-Based Thermodynamics and Kinetics of Molecular Machines: The Importance of Microscopic Reversibility
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-10-11 00:00:00 , DOI: 10.1021/acs.accounts.8b00253 R. Dean Astumian 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2018-10-11 00:00:00 , DOI: 10.1021/acs.accounts.8b00253 R. Dean Astumian 1
Affiliation
|
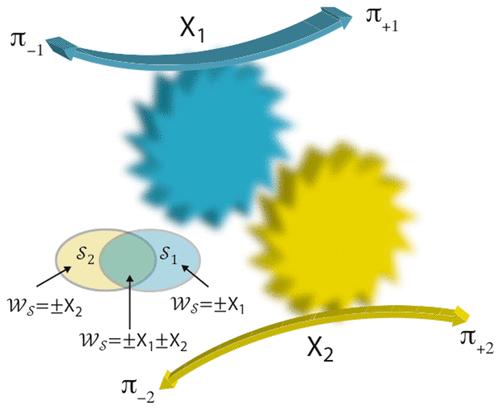
A molecular machine is a nanoscale device that provides a mechanism for coupling energy from two (or more) processes that in the absence of the machine would be independent of one another. Examples include walking of a protein in one direction along a polymeric track (process 1, driving “force” X1 = −F⃗·l⃗) and hydrolyzing ATP (process 2, driving “force” X2 = ΔμATP); or synthesis of ATP (process 1, X1 = −ΔμATP) and transport of protons from the periplasm to the cytoplasm across a membrane (process 2, X2 = ΔμH+); or rotation of a flagellum (process 1, X1 = −torque) and transport of protons across a membrane (process 2, X2 = ΔμH+). In some ways, the function of a molecular machine is similar to that of a macroscopic machine such as a car that couples combustion of gasoline to translational motion. However, the low Reynolds number regime in which molecular machines operate is very different from that relevant for macroscopic machines. Inertia is negligible in comparison to viscous drag, and omnipresent thermal noise causes the machine to undergo continual transition among many states even at thermodynamic equilibrium. Cyclic trajectories among the states of the machine that result in a change in the environment can be broken into two classes: those in which process 1 in either the forward or backward direction ( ) occurs and which thereby exchange work
) occurs and which thereby exchange work  with the environment; and those in which process 2 in either the forward or backward direction (
with the environment; and those in which process 2 in either the forward or backward direction ( ) occurs and which thereby exchange work
) occurs and which thereby exchange work  with the evironment. These two types of trajectories,
with the evironment. These two types of trajectories,  and
and  , overlap, i.e., there are some trajectories in which both process 1 and process 2 occur, and for which the work exchanged is
, overlap, i.e., there are some trajectories in which both process 1 and process 2 occur, and for which the work exchanged is  . The four subclasses of overlap trajectories [(+1,+2), (+1,–2), (−1,+2), (−1,–2)] are the coupled processes. The net probabilities for process 1 and process 2 are designated π+2 – π–2 and π+1 – π–1, respectively. The probabilities
. The four subclasses of overlap trajectories [(+1,+2), (+1,–2), (−1,+2), (−1,–2)] are the coupled processes. The net probabilities for process 1 and process 2 are designated π+2 – π–2 and π+1 – π–1, respectively. The probabilities  for any single trajectory
for any single trajectory  and
and  for its microscopic reverse
for its microscopic reverse  are related by microscopic reversibility (MR),
are related by microscopic reversibility (MR),  , an equality that holds arbitrarily far from thermodynamic equilibrium, i.e., irrespective of the magnitudes of X1 and X2, and where
, an equality that holds arbitrarily far from thermodynamic equilibrium, i.e., irrespective of the magnitudes of X1 and X2, and where  . Using this formalism, we arrive at a remarkably simple and general expression for the rates of the processes,
. Using this formalism, we arrive at a remarkably simple and general expression for the rates of the processes,  , i = 1, 2, where the angle brackets indicate an average over the ensemble of all microscopic reverse trajectories. Stochastic description of coupling is doubtless less familiar than typical mechanical depictions of chemical coupling in terms of ATP induced violent kicks, judo throws, force generation and power-strokes. While the mechanical description of molecular machines is comforting in its familiarity, conclusions based on such a phenomenological perspective are often wrong. Specifically, a “power-stroke” model (i.e., a model based on energy driven “promotion” of a molecular machine to a high energy state followed by directional relaxation to a lower energy state) that has been the focus of mechanistic discussions of biomolecular machines for over a half century is, for catalysis driven molecular machines, incorrect. Instead, the key principle by which catalysis driven motors work is kinetic gating by a mechanism known as an information ratchet. Amazingly, this same principle is that by which catalytic molecular systems undergo adaptation to new steady states while facilitating an exergonic chemical reaction.
, i = 1, 2, where the angle brackets indicate an average over the ensemble of all microscopic reverse trajectories. Stochastic description of coupling is doubtless less familiar than typical mechanical depictions of chemical coupling in terms of ATP induced violent kicks, judo throws, force generation and power-strokes. While the mechanical description of molecular machines is comforting in its familiarity, conclusions based on such a phenomenological perspective are often wrong. Specifically, a “power-stroke” model (i.e., a model based on energy driven “promotion” of a molecular machine to a high energy state followed by directional relaxation to a lower energy state) that has been the focus of mechanistic discussions of biomolecular machines for over a half century is, for catalysis driven molecular machines, incorrect. Instead, the key principle by which catalysis driven motors work is kinetic gating by a mechanism known as an information ratchet. Amazingly, this same principle is that by which catalytic molecular systems undergo adaptation to new steady states while facilitating an exergonic chemical reaction.
中文翻译:

轨迹和基于循环的分子机器的热力学和动力学:微观可逆性的重要性
分子机器是一种纳米级设备,它提供了一种机制,用于耦合来自两个(或多个)过程的能量,而在没有该机器的情况下,该过程将彼此独立。实例包括在一个方向上沿轨道聚合行走的蛋白质的(处理1中,驾驶“力” X 1 = - F⃗ · l⃗)和水解ATP(过程2,驾驶“力” X 2 =Δμ ATP); 或ATP(过程1,X合成1 =-Δμ ATP)和穿过膜从周质至细胞质质子传输(处理2,X 2 =Δμ ħ +); 或鞭毛的旋转(过程1,X 1-Torque =)和穿过膜的质子(过程2,X的运输2 =Δμ ħ +)。在某些方面,分子机器的功能类似于将汽油燃烧与平移运动耦合的宏观机器(例如汽车)的功能。但是,分子机器运行的低雷诺数机制与宏观机器的机制大不相同。与粘性阻力相比,惯性可以忽略不计,并且无处不在的热噪声甚至在热力学平衡下也导致机器在许多状态之间经历连续过渡。导致环境变化的机器状态之间的循环轨迹可以分为两类:进程1处于向前或向后方向( )发生,从而
)发生,从而 与环境交换工作;以及其中
与环境交换工作;以及其中 发生了向前或向后方向()的过程2并由此
发生了向前或向后方向()的过程2并由此 与环境交换工作的过程。这两种类型的轨迹
与环境交换工作的过程。这两种类型的轨迹 和
和 重叠,即,存在一些轨迹,其中过程1和过程2都发生,并且要交换的功为
重叠,即,存在一些轨迹,其中过程1和过程2都发生,并且要交换的功为 。重叠轨迹的四个子类[(+ 1,+ 2),(+ 1,-2),(-1,+ 2),(-1,-2)]是耦合过程。过程1和过程2的净概率分别指定为π +2 –π –2和π +1 –π –1。
。重叠轨迹的四个子类[(+ 1,+ 2),(+ 1,-2),(-1,+ 2),(-1,-2)]是耦合过程。过程1和过程2的净概率分别指定为π +2 –π –2和π +1 –π –1。 任何一条轨迹的概率
任何一条轨迹的概率 和
和 其微观反向
其微观反向 由微观可逆性(MR)的关系,
由微观可逆性(MR)的关系, 即保持从热力学平衡任意远的相等,即,不论X的大小1和X 2,和其中
即保持从热力学平衡任意远的相等,即,不论X的大小1和X 2,和其中 。利用这种形式主义,我们得出一个非常简单和普通的表达对过程的速率,
。利用这种形式主义,我们得出一个非常简单和普通的表达对过程的速率, ,我= 1,2,其中尖括号表示所有微观反向轨迹整体的平均值。就ATP引起的猛烈踢,柔道摔,发力和中风而言,对耦合的随机描述无疑不如对化学耦合的典型机械描述那么熟悉。尽管对分子机器的机械描述在熟悉方面令人感到安慰,但是基于这种现象学观点的结论通常是错误的。具体来说,“动力冲程”模型(即基于能量驱动的分子机器“促进”到高能态,然后定向松弛到低能态的模型)一直是生物分子力学讨论的重点。对于催化驱动的分子机器,半个多世纪的机器是不正确的。反而,催化驱动电机工作的关键原理是通过称为信息棘轮的机制进行动态门控。令人惊讶的是,同样的原理是催化分子系统适应新的稳态,同时促进了强能化学反应。
,我= 1,2,其中尖括号表示所有微观反向轨迹整体的平均值。就ATP引起的猛烈踢,柔道摔,发力和中风而言,对耦合的随机描述无疑不如对化学耦合的典型机械描述那么熟悉。尽管对分子机器的机械描述在熟悉方面令人感到安慰,但是基于这种现象学观点的结论通常是错误的。具体来说,“动力冲程”模型(即基于能量驱动的分子机器“促进”到高能态,然后定向松弛到低能态的模型)一直是生物分子力学讨论的重点。对于催化驱动的分子机器,半个多世纪的机器是不正确的。反而,催化驱动电机工作的关键原理是通过称为信息棘轮的机制进行动态门控。令人惊讶的是,同样的原理是催化分子系统适应新的稳态,同时促进了强能化学反应。
更新日期:2018-10-11
 ) occurs and which thereby exchange work
) occurs and which thereby exchange work  with the environment; and those in which process 2 in either the forward or backward direction (
with the environment; and those in which process 2 in either the forward or backward direction ( ) occurs and which thereby exchange work
) occurs and which thereby exchange work  with the evironment. These two types of trajectories,
with the evironment. These two types of trajectories,  and
and  , overlap, i.e., there are some trajectories in which both process 1 and process 2 occur, and for which the work exchanged is
, overlap, i.e., there are some trajectories in which both process 1 and process 2 occur, and for which the work exchanged is  . The four subclasses of overlap trajectories [(+1,+2), (+1,–2), (−1,+2), (−1,–2)] are the coupled processes. The net probabilities for process 1 and process 2 are designated π+2 – π–2 and π+1 – π–1, respectively. The probabilities
. The four subclasses of overlap trajectories [(+1,+2), (+1,–2), (−1,+2), (−1,–2)] are the coupled processes. The net probabilities for process 1 and process 2 are designated π+2 – π–2 and π+1 – π–1, respectively. The probabilities  for any single trajectory
for any single trajectory  and
and  for its microscopic reverse
for its microscopic reverse  are related by microscopic reversibility (MR),
are related by microscopic reversibility (MR),  , an equality that holds arbitrarily far from thermodynamic equilibrium, i.e., irrespective of the magnitudes of X1 and X2, and where
, an equality that holds arbitrarily far from thermodynamic equilibrium, i.e., irrespective of the magnitudes of X1 and X2, and where  . Using this formalism, we arrive at a remarkably simple and general expression for the rates of the processes,
. Using this formalism, we arrive at a remarkably simple and general expression for the rates of the processes,  , i = 1, 2, where the angle brackets indicate an average over the ensemble of all microscopic reverse trajectories. Stochastic description of coupling is doubtless less familiar than typical mechanical depictions of chemical coupling in terms of ATP induced violent kicks, judo throws, force generation and power-strokes. While the mechanical description of molecular machines is comforting in its familiarity, conclusions based on such a phenomenological perspective are often wrong. Specifically, a “power-stroke” model (i.e., a model based on energy driven “promotion” of a molecular machine to a high energy state followed by directional relaxation to a lower energy state) that has been the focus of mechanistic discussions of biomolecular machines for over a half century is, for catalysis driven molecular machines, incorrect. Instead, the key principle by which catalysis driven motors work is kinetic gating by a mechanism known as an information ratchet. Amazingly, this same principle is that by which catalytic molecular systems undergo adaptation to new steady states while facilitating an exergonic chemical reaction.
, i = 1, 2, where the angle brackets indicate an average over the ensemble of all microscopic reverse trajectories. Stochastic description of coupling is doubtless less familiar than typical mechanical depictions of chemical coupling in terms of ATP induced violent kicks, judo throws, force generation and power-strokes. While the mechanical description of molecular machines is comforting in its familiarity, conclusions based on such a phenomenological perspective are often wrong. Specifically, a “power-stroke” model (i.e., a model based on energy driven “promotion” of a molecular machine to a high energy state followed by directional relaxation to a lower energy state) that has been the focus of mechanistic discussions of biomolecular machines for over a half century is, for catalysis driven molecular machines, incorrect. Instead, the key principle by which catalysis driven motors work is kinetic gating by a mechanism known as an information ratchet. Amazingly, this same principle is that by which catalytic molecular systems undergo adaptation to new steady states while facilitating an exergonic chemical reaction.
中文翻译:

轨迹和基于循环的分子机器的热力学和动力学:微观可逆性的重要性
分子机器是一种纳米级设备,它提供了一种机制,用于耦合来自两个(或多个)过程的能量,而在没有该机器的情况下,该过程将彼此独立。实例包括在一个方向上沿轨道聚合行走的蛋白质的(处理1中,驾驶“力” X 1 = - F⃗ · l⃗)和水解ATP(过程2,驾驶“力” X 2 =Δμ ATP); 或ATP(过程1,X合成1 =-Δμ ATP)和穿过膜从周质至细胞质质子传输(处理2,X 2 =Δμ ħ +); 或鞭毛的旋转(过程1,X 1-Torque =)和穿过膜的质子(过程2,X的运输2 =Δμ ħ +)。在某些方面,分子机器的功能类似于将汽油燃烧与平移运动耦合的宏观机器(例如汽车)的功能。但是,分子机器运行的低雷诺数机制与宏观机器的机制大不相同。与粘性阻力相比,惯性可以忽略不计,并且无处不在的热噪声甚至在热力学平衡下也导致机器在许多状态之间经历连续过渡。导致环境变化的机器状态之间的循环轨迹可以分为两类:进程1处于向前或向后方向(
 )发生,从而
)发生,从而 与环境交换工作;以及其中
与环境交换工作;以及其中 发生了向前或向后方向()的过程2并由此
发生了向前或向后方向()的过程2并由此 与环境交换工作的过程。这两种类型的轨迹
与环境交换工作的过程。这两种类型的轨迹 和
和 重叠,即,存在一些轨迹,其中过程1和过程2都发生,并且要交换的功为
重叠,即,存在一些轨迹,其中过程1和过程2都发生,并且要交换的功为 。重叠轨迹的四个子类[(+ 1,+ 2),(+ 1,-2),(-1,+ 2),(-1,-2)]是耦合过程。过程1和过程2的净概率分别指定为π +2 –π –2和π +1 –π –1。
。重叠轨迹的四个子类[(+ 1,+ 2),(+ 1,-2),(-1,+ 2),(-1,-2)]是耦合过程。过程1和过程2的净概率分别指定为π +2 –π –2和π +1 –π –1。 任何一条轨迹的概率
任何一条轨迹的概率 和
和 其微观反向
其微观反向 由微观可逆性(MR)的关系,
由微观可逆性(MR)的关系, 即保持从热力学平衡任意远的相等,即,不论X的大小1和X 2,和其中
即保持从热力学平衡任意远的相等,即,不论X的大小1和X 2,和其中 。利用这种形式主义,我们得出一个非常简单和普通的表达对过程的速率,
。利用这种形式主义,我们得出一个非常简单和普通的表达对过程的速率, ,我= 1,2,其中尖括号表示所有微观反向轨迹整体的平均值。就ATP引起的猛烈踢,柔道摔,发力和中风而言,对耦合的随机描述无疑不如对化学耦合的典型机械描述那么熟悉。尽管对分子机器的机械描述在熟悉方面令人感到安慰,但是基于这种现象学观点的结论通常是错误的。具体来说,“动力冲程”模型(即基于能量驱动的分子机器“促进”到高能态,然后定向松弛到低能态的模型)一直是生物分子力学讨论的重点。对于催化驱动的分子机器,半个多世纪的机器是不正确的。反而,催化驱动电机工作的关键原理是通过称为信息棘轮的机制进行动态门控。令人惊讶的是,同样的原理是催化分子系统适应新的稳态,同时促进了强能化学反应。
,我= 1,2,其中尖括号表示所有微观反向轨迹整体的平均值。就ATP引起的猛烈踢,柔道摔,发力和中风而言,对耦合的随机描述无疑不如对化学耦合的典型机械描述那么熟悉。尽管对分子机器的机械描述在熟悉方面令人感到安慰,但是基于这种现象学观点的结论通常是错误的。具体来说,“动力冲程”模型(即基于能量驱动的分子机器“促进”到高能态,然后定向松弛到低能态的模型)一直是生物分子力学讨论的重点。对于催化驱动的分子机器,半个多世纪的机器是不正确的。反而,催化驱动电机工作的关键原理是通过称为信息棘轮的机制进行动态门控。令人惊讶的是,同样的原理是催化分子系统适应新的稳态,同时促进了强能化学反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号