当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Pot Biocombinatorial Synthesis of Herbicidal Thaxtomins
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acscatal.8b03317 Guangde Jiang 1 , Ran Zuo 1 , Yi Zhang 1 , Magan M. Powell 1 , Peilan Zhang 1 , Sarah M. Hylton 1 , Rosemary Loria 2 , Yousong Ding 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acscatal.8b03317 Guangde Jiang 1 , Ran Zuo 1 , Yi Zhang 1 , Magan M. Powell 1 , Peilan Zhang 1 , Sarah M. Hylton 1 , Rosemary Loria 2 , Yousong Ding 1
Affiliation
|
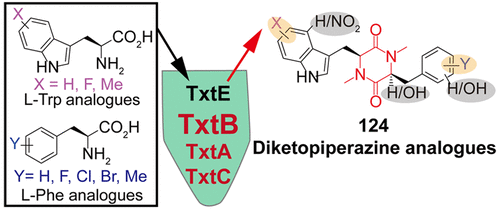
Thaxtomins are a group of phytotoxic diketopiperazines produced by tens of plant pathogenic Streptomyces strains and have received considerable attention as bioherbicide. To synthesize thaxtomin analogue libraries for herbicide development, we here develop an in vitro biocombinatorial approach. We first prepared two recombinant single-module multifunctional nonribosomal peptide synthetases (NRPSs), TxtA and TxtB. Biochemical studies revealed that TxtA and TxtB tolerated small substituents on the aromatic moiety of l-Phe and l-Trp, respectively. Intriguingly, TxtB showed a control of the substrate scopes of TxtA and a previously characterized, pathway-specific nitration promoting P450 TxtE. We further demonstrated that the methyltransferase (MT) domain of TxtA and TxtB posed a minimal influence on the assembly of the diketopiperazine core by other domains of these two enzymes, providing a way for structural diversification. The pathway-specific bifunctional P450 TxtC was then recombinantly produced in Escherichia coli after being fused with the reductase domain of P450BM3. Biochemical and kinetic studies indicated that this self-sufficient biocatalyst promoted two hydroxylation reactions first on the aliphatic C14 and then the aromatic C20 of thaxtomin D to sequentially produce thaxtomin B and A. Using these enzymes, a one-pot biocatalytic reaction was developed to synthesize 124 thaxtomin analogues, whose structures were validated in high-resolution MS, tandem MS, and sometimes 1D and 2D NMR analysis. Select thaxtomin analogues showed potent herbicidal activities in radish seedling assay. This work demonstrated the feasibility of biocombinatorial synthesis in the creation of natural product-like libraries and provided useful insights into the model of diketopiperazine structural diversity generation in nature, aiding the development of bioactive peptidic compounds in general.
中文翻译:

一锅生物组合法合成除草素
thaxtomins是一组由数十种植物致病性链霉菌菌株产生的具有植物毒性的二酮哌嗪类,已作为生物除草剂受到了广泛关注。为了合成除草剂开发的thaxtomin类似物库,我们在这里开发了一种体外生物组合方法。我们首先准备了两个重组单模块多功能非核糖体肽合成酶(NRPSs),TxtA和TxtB。生化研究表明,TxtA和TxtB可以耐受1- Phe和l的芳族部分上的小取代基-Trp,分别。有趣的是,TxtB显示了对TxtA底物范围的控制以及先前表征的促进P450 TxtE的途径特异性硝化作用。我们进一步证明,TxtA和TxtB的甲基转移酶(MT)结构域对这两种酶的其他结构域对二酮哌嗪核心的组装影响最小,为结构多样化提供了一种途径。然后在大肠杆菌中重组生产途径特异性双功能P450 TxtC与P450BM3的还原酶结构域融合后。生化和动力学研究表明,这种自给自足的生物催化剂首先促进了thaxtomin D的脂族C14,然后在芳香族C20上的两个羟基化反应,从而依次产生thaxtomin B和A。 124种thaxtomin类似物,其结构已通过高分辨率MS,串联MS以及有时的1D和2D NMR分析得到了验证。精选的Thaxtomin类似物在萝卜幼苗测定中显示出强力的除草活性。这项工作证明了在创建类似天然产物的文库中生物组合合成的可行性,并为深入了解自然界中二酮哌嗪结构多样性产生的模型提供了有用的见识,
更新日期:2018-10-10
中文翻译:

一锅生物组合法合成除草素
thaxtomins是一组由数十种植物致病性链霉菌菌株产生的具有植物毒性的二酮哌嗪类,已作为生物除草剂受到了广泛关注。为了合成除草剂开发的thaxtomin类似物库,我们在这里开发了一种体外生物组合方法。我们首先准备了两个重组单模块多功能非核糖体肽合成酶(NRPSs),TxtA和TxtB。生化研究表明,TxtA和TxtB可以耐受1- Phe和l的芳族部分上的小取代基-Trp,分别。有趣的是,TxtB显示了对TxtA底物范围的控制以及先前表征的促进P450 TxtE的途径特异性硝化作用。我们进一步证明,TxtA和TxtB的甲基转移酶(MT)结构域对这两种酶的其他结构域对二酮哌嗪核心的组装影响最小,为结构多样化提供了一种途径。然后在大肠杆菌中重组生产途径特异性双功能P450 TxtC与P450BM3的还原酶结构域融合后。生化和动力学研究表明,这种自给自足的生物催化剂首先促进了thaxtomin D的脂族C14,然后在芳香族C20上的两个羟基化反应,从而依次产生thaxtomin B和A。 124种thaxtomin类似物,其结构已通过高分辨率MS,串联MS以及有时的1D和2D NMR分析得到了验证。精选的Thaxtomin类似物在萝卜幼苗测定中显示出强力的除草活性。这项工作证明了在创建类似天然产物的文库中生物组合合成的可行性,并为深入了解自然界中二酮哌嗪结构多样性产生的模型提供了有用的见识,










































 京公网安备 11010802027423号
京公网安备 11010802027423号