Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Neural blastocyst complementation enables mouse forebrain organogenesis
Nature ( IF 50.5 ) Pub Date : 2018-10-10 , DOI: 10.1038/s41586-018-0586-0 Amelia N Chang 1 , Zhuoyi Liang 1 , Hai-Qiang Dai 1 , Aimee M Chapdelaine-Williams 1 , Nick Andrews 2 , Roderick T Bronson 3 , Bjoern Schwer 4 , Frederick W Alt 1
Nature ( IF 50.5 ) Pub Date : 2018-10-10 , DOI: 10.1038/s41586-018-0586-0 Amelia N Chang 1 , Zhuoyi Liang 1 , Hai-Qiang Dai 1 , Aimee M Chapdelaine-Williams 1 , Nick Andrews 2 , Roderick T Bronson 3 , Bjoern Schwer 4 , Frederick W Alt 1
Affiliation
|
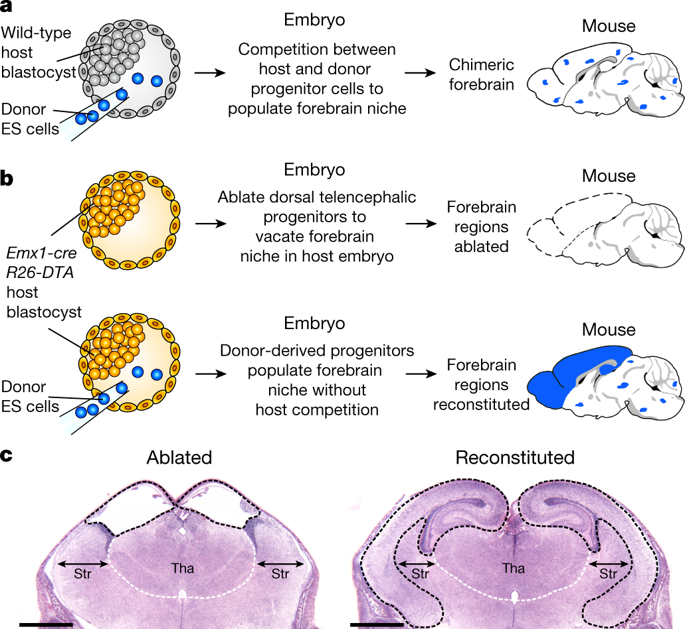
Genetically modified mice are commonly generated by the microinjection of pluripotent embryonic stem (ES) cells into wild-type host blastocysts1, producing chimeric progeny that require breeding for germline transmission and homozygosity of modified alleles. As an alternative approach and to facilitate studies of the immune system, we previously developed RAG2-deficient blastocyst complementation2. Because RAG2-deficient mice cannot undergo V(D)J recombination, they do not develop B or T lineage cells beyond the progenitor stage2: injecting RAG2-sufficient donor ES cells into RAG2-deficient blastocysts generates somatic chimaeras in which all mature lymphocytes derive from donor ES cells. This enables analysis, in mature lymphocytes, of the functions of genes that are required more generally for mouse development3. Blastocyst complementation has been extended to pancreas organogenesis4, and used to generate several other tissues or organs5–10, but an equivalent approach for brain organogenesis has not yet been achieved. Here we describe neural blastocyst complementation (NBC), which can be used to study the development and function of specific forebrain regions. NBC involves targeted ablation, mediated by diphtheria toxin subunit A, of host-derived dorsal telencephalic progenitors during development. This ablation creates a vacant forebrain niche in host embryos that results in agenesis of the cerebral cortex and hippocampus. Injection of donor ES cells into blastocysts with forebrain-specific targeting of diphtheria toxin subunit A enables donor-derived dorsal telencephalic progenitors to populate the vacant niche in the host embryos, giving rise to neocortices and hippocampi that are morphologically and neurologically normal with respect to learning and memory formation. Moreover, doublecortin-deficient ES cells—generated via a CRISPR–Cas9 approach—produced NBC chimaeras that faithfully recapitulated the phenotype of conventional, germline doublecortin-deficient mice. We conclude that NBC is a rapid and efficient approach to generate complex mouse models for studying forebrain functions; this approach could more broadly facilitate organogenesis based on blastocyst complementation.Neural blastocyst complementation creates a vacant forebrain niche in host embryos that can be populated by donor embryonic stem cell-derived dorsal telencephalic progenitors, resulting in a mouse brain organogenesis model.
中文翻译:

神经囊胚互补使小鼠前脑器官发生
转基因小鼠通常是通过将多能胚胎干 (ES) 细胞显微注射到野生型宿主囊胚中来产生的,从而产生嵌合后代,这些后代需要繁殖以进行种系传递和修饰等位基因的纯合性。作为一种替代方法并促进免疫系统的研究,我们以前开发了 RAG2 缺陷的囊胚互补 2。因为 RAG2 缺陷小鼠不能进行 V(D)J 重组,所以它们不会在祖细胞阶段后发育 B 或 T 谱系细胞2:将 RAG2 足够的供体 ES 细胞注射到 RAG2 缺陷的囊胚中会产生体细胞嵌合体,其中所有成熟的淋巴细胞都来自供体ES细胞。这使得在成熟淋巴细胞中分析小鼠发育更普遍需要的基因功能成为可能。囊胚互补已扩展到胰腺器官发生 4,并用于生成其他几种组织或器官 5-10,但尚未实现脑器官发生的等效方法。在这里,我们描述了神经囊胚互补 (NBC),可用于研究特定前脑区域的发育和功能。NBC 涉及在发育过程中由宿主来源的背侧端脑祖细胞的白喉毒素亚基 A 介导的靶向消融。这种消融在宿主胚胎中创造了一个空的前脑生态位,导致大脑皮层和海马体发育不全。将供体 ES 细胞注射到具有前脑特异性靶向白喉毒素亚基 A 的囊胚中,使供体来源的背侧端脑祖细胞能够填充宿主胚胎中的空位,产生在学习和记忆形成方面在形态学和神经学上正常的新皮质和海马体。此外,双皮质素缺陷型 ES 细胞——通过 CRISPR-Cas9 方法产生——产生的 NBC 嵌合体忠实地再现了传统的、种系双皮质素缺陷型小鼠的表型。我们得出结论,NBC 是一种快速有效的方法,可以生成用于研究前脑功能的复杂小鼠模型;这种方法可以更广泛地促进基于囊胚互补的器官发生。神经囊胚互补在宿主胚胎中创造了一个空的前脑生态位,可以由供体胚胎干细胞衍生的背侧端脑祖细胞填充,从而形成小鼠大脑器官发生模型。
更新日期:2018-10-10
中文翻译:

神经囊胚互补使小鼠前脑器官发生
转基因小鼠通常是通过将多能胚胎干 (ES) 细胞显微注射到野生型宿主囊胚中来产生的,从而产生嵌合后代,这些后代需要繁殖以进行种系传递和修饰等位基因的纯合性。作为一种替代方法并促进免疫系统的研究,我们以前开发了 RAG2 缺陷的囊胚互补 2。因为 RAG2 缺陷小鼠不能进行 V(D)J 重组,所以它们不会在祖细胞阶段后发育 B 或 T 谱系细胞2:将 RAG2 足够的供体 ES 细胞注射到 RAG2 缺陷的囊胚中会产生体细胞嵌合体,其中所有成熟的淋巴细胞都来自供体ES细胞。这使得在成熟淋巴细胞中分析小鼠发育更普遍需要的基因功能成为可能。囊胚互补已扩展到胰腺器官发生 4,并用于生成其他几种组织或器官 5-10,但尚未实现脑器官发生的等效方法。在这里,我们描述了神经囊胚互补 (NBC),可用于研究特定前脑区域的发育和功能。NBC 涉及在发育过程中由宿主来源的背侧端脑祖细胞的白喉毒素亚基 A 介导的靶向消融。这种消融在宿主胚胎中创造了一个空的前脑生态位,导致大脑皮层和海马体发育不全。将供体 ES 细胞注射到具有前脑特异性靶向白喉毒素亚基 A 的囊胚中,使供体来源的背侧端脑祖细胞能够填充宿主胚胎中的空位,产生在学习和记忆形成方面在形态学和神经学上正常的新皮质和海马体。此外,双皮质素缺陷型 ES 细胞——通过 CRISPR-Cas9 方法产生——产生的 NBC 嵌合体忠实地再现了传统的、种系双皮质素缺陷型小鼠的表型。我们得出结论,NBC 是一种快速有效的方法,可以生成用于研究前脑功能的复杂小鼠模型;这种方法可以更广泛地促进基于囊胚互补的器官发生。神经囊胚互补在宿主胚胎中创造了一个空的前脑生态位,可以由供体胚胎干细胞衍生的背侧端脑祖细胞填充,从而形成小鼠大脑器官发生模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号