Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
IRE1α–XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity
Nature ( IF 64.8 ) Pub Date : 2018-10-01 , DOI: 10.1038/s41586-018-0597-x Minkyung Song 1, 2, 3 , Tito A Sandoval 2, 3 , Chang-Suk Chae 2, 3 , Sahil Chopra 1, 2, 3 , Chen Tan 2, 3 , Melanie R Rutkowski 4 , Mahesh Raundhal 5, 6 , Ricardo A Chaurio 7 , Kyle K Payne 7 , Csaba Konrad 8 , Sarah E Bettigole 9 , Hee Rae Shin 9 , Michael J P Crowley 1 , Juan P Cerliani 10 , Andrew V Kossenkov 11 , Ievgen Motorykin 12 , Sheng Zhang 12 , Giovanni Manfredi 8 , Dmitriy Zamarin 13 , Kevin Holcomb 2, 3 , Paulo C Rodriguez 7 , Gabriel A Rabinovich 10, 14 , Jose R Conejo-Garcia 7 , Laurie H Glimcher 5, 6 , Juan R Cubillos-Ruiz 1, 2, 3
Nature ( IF 64.8 ) Pub Date : 2018-10-01 , DOI: 10.1038/s41586-018-0597-x Minkyung Song 1, 2, 3 , Tito A Sandoval 2, 3 , Chang-Suk Chae 2, 3 , Sahil Chopra 1, 2, 3 , Chen Tan 2, 3 , Melanie R Rutkowski 4 , Mahesh Raundhal 5, 6 , Ricardo A Chaurio 7 , Kyle K Payne 7 , Csaba Konrad 8 , Sarah E Bettigole 9 , Hee Rae Shin 9 , Michael J P Crowley 1 , Juan P Cerliani 10 , Andrew V Kossenkov 11 , Ievgen Motorykin 12 , Sheng Zhang 12 , Giovanni Manfredi 8 , Dmitriy Zamarin 13 , Kevin Holcomb 2, 3 , Paulo C Rodriguez 7 , Gabriel A Rabinovich 10, 14 , Jose R Conejo-Garcia 7 , Laurie H Glimcher 5, 6 , Juan R Cubillos-Ruiz 1, 2, 3
Affiliation
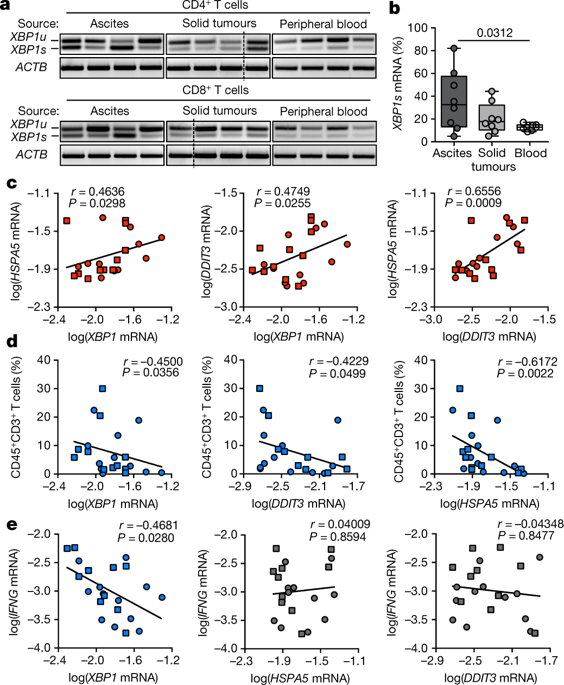
|
Tumours evade immune control by creating hostile microenvironments that perturb T cell metabolism and effector function1–4. However, it remains unclear how intra-tumoral T cells integrate and interpret metabolic stress signals. Here we report that ovarian cancer—an aggressive malignancy that is refractory to standard treatments and current immunotherapies5–8—induces endoplasmic reticulum stress and activates the IRE1α–XBP1 arm of the unfolded protein response9,10 in T cells to control their mitochondrial respiration and anti-tumour function. In T cells isolated from specimens collected from patients with ovarian cancer, upregulation of XBP1 was associated with decreased infiltration of T cells into tumours and with reduced IFNG mRNA expression. Malignant ascites fluid obtained from patients with ovarian cancer inhibited glucose uptake and caused N-linked protein glycosylation defects in T cells, which triggered IRE1α–XBP1 activation that suppressed mitochondrial activity and IFNγ production. Mechanistically, induction of XBP1 regulated the abundance of glutamine carriers and thus limited the influx of glutamine that is necessary to sustain mitochondrial respiration in T cells under glucose-deprived conditions. Restoring N-linked protein glycosylation, abrogating IRE1α–XBP1 activation or enforcing expression of glutamine transporters enhanced mitochondrial respiration in human T cells exposed to ovarian cancer ascites. XBP1-deficient T cells in the metastatic ovarian cancer milieu exhibited global transcriptional reprogramming and improved effector capacity. Accordingly, mice that bear ovarian cancer and lack XBP1 selectively in T cells demonstrate superior anti-tumour immunity, delayed malignant progression and increased overall survival. Controlling endoplasmic reticulum stress or targeting IRE1α–XBP1 signalling may help to restore the metabolic fitness and anti-tumour capacity of T cells in cancer hosts.In human and mouse models of ovarian cancer, endoplasmic reticulum stress and the activation of the IRE1α–XBP1 pathway decreases the metabolic fitness of T cells and limits their anti-tumour functions.
中文翻译:

IRE1α-XBP1通过调节线粒体活性控制卵巢癌中的T细胞功能
肿瘤通过创造扰乱 T 细胞代谢和效应器功能的敌对微环境来逃避免疫控制1-4。然而,目前尚不清楚肿瘤内 T 细胞如何整合和解释代谢应激信号。在这里,我们报告卵巢癌——一种侵袭性恶性肿瘤,对标准治疗和当前的免疫疗法5-8 无效——诱导内质网应激并激活 T 细胞中未折叠蛋白反应的 IRE1α-XBP1 臂 9,10 以控制它们的线粒体呼吸和抗- 肿瘤功能。在从卵巢癌患者的标本中分离出的 T 细胞中,XBP1 的上调与 T 细胞向肿瘤的浸润减少和 IFNG mRNA 表达降低有关。从卵巢癌患者体内获得的恶性腹水会抑制葡萄糖摄取并导致 T 细胞中的 N 连接蛋白糖基化缺陷,从而触发 IRE1α-XBP1 激活,从而抑制线粒体活性和 IFNγ 的产生。从机制上讲,XBP1 的诱导调节了谷氨酰胺载体的丰度,从而限制了谷氨酰胺的流入,而谷氨酰胺是在葡萄糖剥夺条件下维持 T 细胞线粒体呼吸所必需的。恢复 N 连接蛋白糖基化、取消 IRE1α-XBP1 激活或强制表达谷氨酰胺转运蛋白可增强暴露于卵巢癌腹水的人类 T 细胞的线粒体呼吸。转移性卵巢癌环境中的 XBP1 缺陷 T 细胞表现出整体转录重编程和改善的效应器能力。因此,患有卵巢癌且 T 细胞中选择性缺乏 XBP1 的小鼠表现出优异的抗肿瘤免疫力、延迟恶性进展和增加总生存率。控制内质网应激或靶向 IRE1α-XBP1 信号可能有助于恢复癌症宿主中 T 细胞的代谢适应性和抗肿瘤能力。在卵巢癌的人和小鼠模型中,内质网应激和 IRE1α-XBP1 通路的激活降低 T 细胞的代谢适应性并限制其抗肿瘤功能。
更新日期:2018-10-01
中文翻译:

IRE1α-XBP1通过调节线粒体活性控制卵巢癌中的T细胞功能
肿瘤通过创造扰乱 T 细胞代谢和效应器功能的敌对微环境来逃避免疫控制1-4。然而,目前尚不清楚肿瘤内 T 细胞如何整合和解释代谢应激信号。在这里,我们报告卵巢癌——一种侵袭性恶性肿瘤,对标准治疗和当前的免疫疗法5-8 无效——诱导内质网应激并激活 T 细胞中未折叠蛋白反应的 IRE1α-XBP1 臂 9,10 以控制它们的线粒体呼吸和抗- 肿瘤功能。在从卵巢癌患者的标本中分离出的 T 细胞中,XBP1 的上调与 T 细胞向肿瘤的浸润减少和 IFNG mRNA 表达降低有关。从卵巢癌患者体内获得的恶性腹水会抑制葡萄糖摄取并导致 T 细胞中的 N 连接蛋白糖基化缺陷,从而触发 IRE1α-XBP1 激活,从而抑制线粒体活性和 IFNγ 的产生。从机制上讲,XBP1 的诱导调节了谷氨酰胺载体的丰度,从而限制了谷氨酰胺的流入,而谷氨酰胺是在葡萄糖剥夺条件下维持 T 细胞线粒体呼吸所必需的。恢复 N 连接蛋白糖基化、取消 IRE1α-XBP1 激活或强制表达谷氨酰胺转运蛋白可增强暴露于卵巢癌腹水的人类 T 细胞的线粒体呼吸。转移性卵巢癌环境中的 XBP1 缺陷 T 细胞表现出整体转录重编程和改善的效应器能力。因此,患有卵巢癌且 T 细胞中选择性缺乏 XBP1 的小鼠表现出优异的抗肿瘤免疫力、延迟恶性进展和增加总生存率。控制内质网应激或靶向 IRE1α-XBP1 信号可能有助于恢复癌症宿主中 T 细胞的代谢适应性和抗肿瘤能力。在卵巢癌的人和小鼠模型中,内质网应激和 IRE1α-XBP1 通路的激活降低 T 细胞的代谢适应性并限制其抗肿瘤功能。



























 京公网安备 11010802027423号
京公网安备 11010802027423号