当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of E-3-(arylmethylidene)-5-(alkyl/aryl)-2(3H)-furanones by sequential hydroacyloxylation-Mizoroki–Heck reactions of iodoalkynes
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-10-10 , DOI: 10.1039/c8ob02063a Gopinathan Muthusamy 1, 2, 3, 4 , Sunil V. Pansare 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-10-10 , DOI: 10.1039/c8ob02063a Gopinathan Muthusamy 1, 2, 3, 4 , Sunil V. Pansare 1, 2, 3, 4
Affiliation
|
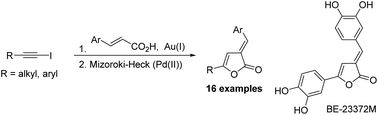
A modular, stereoselective synthesis of E-3-(arylidene)-5-(alkyl/aryl)-2(3H)-furanones was developed. The methodology features regioselective addition of β-aryl acrylic acids to iodoacetylenes to furnish the Z-acyloxy iodoalkenes. A stereoselective 5-exo-trig Mizoroki–Heck reaction of the acyloxy iodoalkenes generates the target E-2(3H)-furanones. The approach was applied in a formal synthesis of the naturally occurring kinase inhibitor BE-23372M.
中文翻译:

E -3-(芳基亚甲基)-5-(烷基/芳基)-2(3 H)-呋喃酮的立体选择性合成通过碘代炔烃的连续氢酰氧基化-Mizoroki-Heck反应
开发了模块化的E -3-(亚芳基)-5-(烷基/芳基)-2(3 H)-呋喃酮的立体选择性合成方法。该方法的特征在于将β-芳基丙烯酸的区域选择性加成到碘乙炔中以提供Z-酰氧基碘烯烃。酰氧基碘代烯烃的立体选择性5 -exo-trig Mizoroki-Heck反应生成目标E -2(3 H)-呋喃酮。该方法被用于天然存在的激酶抑制剂BE-23372M的正式合成中。
更新日期:2018-11-02
中文翻译:

E -3-(芳基亚甲基)-5-(烷基/芳基)-2(3 H)-呋喃酮的立体选择性合成通过碘代炔烃的连续氢酰氧基化-Mizoroki-Heck反应
开发了模块化的E -3-(亚芳基)-5-(烷基/芳基)-2(3 H)-呋喃酮的立体选择性合成方法。该方法的特征在于将β-芳基丙烯酸的区域选择性加成到碘乙炔中以提供Z-酰氧基碘烯烃。酰氧基碘代烯烃的立体选择性5 -exo-trig Mizoroki-Heck反应生成目标E -2(3 H)-呋喃酮。该方法被用于天然存在的激酶抑制剂BE-23372M的正式合成中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号