当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
G-Quadruplex-Based Nanoscale Coordination Polymers to Modulate Tumor Hypoxia and Achieve Nuclear-Targeted Drug Delivery for Enhanced Photodynamic Therapy
Nano Letters ( IF 9.6 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acs.nanolett.8b02732 Yu Yang 1 , Wenjun Zhu 2 , Liangzhu Feng 2 , Yu Chao 2 , Xuan Yi 3 , Ziliang Dong 2 , Kai Yang 3 , Weihong Tan 4 , Zhuang Liu 2 , Meiwan Chen 1
Nano Letters ( IF 9.6 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1021/acs.nanolett.8b02732 Yu Yang 1 , Wenjun Zhu 2 , Liangzhu Feng 2 , Yu Chao 2 , Xuan Yi 3 , Ziliang Dong 2 , Kai Yang 3 , Weihong Tan 4 , Zhuang Liu 2 , Meiwan Chen 1
Affiliation
|
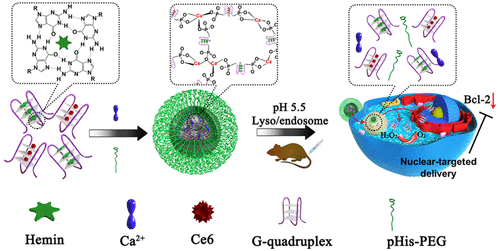
Photodynamic therapy (PDT) is a light-triggered therapy used to kill cancer cells by producing reactive oxygen species (ROS). Herein, a new kind of DNA nanostructure based on the coordination between calcium ions (Ca2+) and AS1411 DNA G quadruplexes to form nanoscale coordination polymers (NCPs) is developed via a simple method. Both chlorine e6 (Ce6), a photosensitizer, and hemin, an iron-containing porphyrin, can be inserted into the G-quadruplex structure in the obtained NCPs. With further polyethylene glycol (PEG) modification, we obtain Ca-AS1411/Ce6/[email protected] (CACH-PEG) NCP nanostructure that enables the intranuclear transport of photosensitizer Ce6 to generate ROS inside cell nuclei that are the most vulnerable to ROS. Meanwhile, the inhibition of antiapoptotic protein B-cell lymphoma 2 (Bcl-2) expression by AS1411 allows for greatly improved PDT-induced cell apoptosis. Furthermore, the catalase-mimicking DNAzyme function of G-quadruplexes and hemin in those NCPs could decompose tumor endogenous H2O2 to in situ generate oxygen so as to further enhance PDT by overcoming the hypoxia-associated resistance. This work develops a simple yet general method with which to fabricate DNA-based NCPs and presents an interesting concept of a nanoscale drug-delivery system that could achieve the intranuclear delivery of photosensitizers, the down-regulation of anti-apoptotic proteins, and the modulation of the unfavorable tumor microenvironment simultaneously for improved cancer therapy.
中文翻译:

基于G-四链体的纳米级配位聚合物可调节肿瘤缺氧并实现核靶向药物递送,从而增强光动力疗法。
光动力疗法(PDT)是一种光触发疗法,用于通过产生活性氧(ROS)杀死癌细胞。在这里,一种基于钙离子(Ca 2+)和AS1411 DNA G四链体形成纳米级配位聚合物(NCP)的方法很简单。可以将光敏剂氯e6(Ce6)和含铁卟啉血红素(hemin)插入获得的NCP中的G-四链体结构中。经过进一步的聚乙二醇(PEG)修饰,我们获得了Ca-AS1411 / Ce6 / [电子邮件保护](CACH-PEG)NCP纳米结构,该结构可使光敏剂Ce6的核内传输在细胞核内产生最易受ROS破坏的ROS。同时,AS1411对抗凋亡蛋白B细胞淋巴瘤2(Bcl-2)表达的抑制作用可大大改善PDT诱导的细胞凋亡。此外,G-四链的过氧化氢酶模拟DNA核酶功能和氯高铁血红素在那些国家联络可以分解肿瘤的内源性ħ 2 ö2克服了与缺氧相关的抵抗力,从而原位产生氧气,从而进一步增强了PDT。这项工作开发了一种简单而通用的方法来制造基于DNA的NCP,并提出了一个有趣的概念,即纳米级药物递送系统可以实现光敏剂的核内递送,抗凋亡蛋白的下调以及调节。同时观察不利的肿瘤微环境,以改善癌症治疗。
更新日期:2018-10-10
中文翻译:

基于G-四链体的纳米级配位聚合物可调节肿瘤缺氧并实现核靶向药物递送,从而增强光动力疗法。
光动力疗法(PDT)是一种光触发疗法,用于通过产生活性氧(ROS)杀死癌细胞。在这里,一种基于钙离子(Ca 2+)和AS1411 DNA G四链体形成纳米级配位聚合物(NCP)的方法很简单。可以将光敏剂氯e6(Ce6)和含铁卟啉血红素(hemin)插入获得的NCP中的G-四链体结构中。经过进一步的聚乙二醇(PEG)修饰,我们获得了Ca-AS1411 / Ce6 / [电子邮件保护](CACH-PEG)NCP纳米结构,该结构可使光敏剂Ce6的核内传输在细胞核内产生最易受ROS破坏的ROS。同时,AS1411对抗凋亡蛋白B细胞淋巴瘤2(Bcl-2)表达的抑制作用可大大改善PDT诱导的细胞凋亡。此外,G-四链的过氧化氢酶模拟DNA核酶功能和氯高铁血红素在那些国家联络可以分解肿瘤的内源性ħ 2 ö2克服了与缺氧相关的抵抗力,从而原位产生氧气,从而进一步增强了PDT。这项工作开发了一种简单而通用的方法来制造基于DNA的NCP,并提出了一个有趣的概念,即纳米级药物递送系统可以实现光敏剂的核内递送,抗凋亡蛋白的下调以及调节。同时观察不利的肿瘤微环境,以改善癌症治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号