当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of a lysine 4-hydroxylase from the glidobactin biosynthesis and evaluation of its biocatalytic potential.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2019-02-13 , DOI: 10.1039/c8ob02054j Alexander Amatuni 1 , Hans Renata
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2019-02-13 , DOI: 10.1039/c8ob02054j Alexander Amatuni 1 , Hans Renata
Affiliation
|
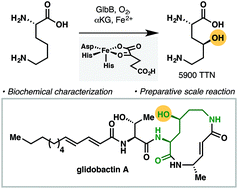
We present the functional characterization of GlbB, a lysine 4-hydroxylase from the glidobactin biosynthetic gene cluster. Despite its narrow substrate specificity, GlbB is able to catalyze the hydroxylation of l-lysine with excellent total turnover number and complete regio- and diastereoselectivity. The synthetic utility of GlbB is illustrated by its use in the efficient preparation of a key dipeptide fragment of glidobactin.
中文翻译:

从glidobactin生物合成中鉴定赖氨酸4-羟化酶并评估其生物催化潜力。
我们目前的功能表征GlbB,从glidobactin生物合成基因簇的赖氨酸4-羟化酶。尽管其底物特异性狭窄,但是GlbB能够以优异的总周转数以及完全的区域和非对映选择性催化L-赖氨酸的羟基化。GlbB在有效制备格列葡汀的关键二肽片段中的应用说明了GlbB的合成效用。
更新日期:2019-02-14
中文翻译:

从glidobactin生物合成中鉴定赖氨酸4-羟化酶并评估其生物催化潜力。
我们目前的功能表征GlbB,从glidobactin生物合成基因簇的赖氨酸4-羟化酶。尽管其底物特异性狭窄,但是GlbB能够以优异的总周转数以及完全的区域和非对映选择性催化L-赖氨酸的羟基化。GlbB在有效制备格列葡汀的关键二肽片段中的应用说明了GlbB的合成效用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号