Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An integrated adipose-tissue-on-chip nanoplasmonic biosensing platform for investigating obesity-associated inflammation†
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1039/c8lc00605a Jingyi Zhu 1, 2, 3, 4 , Jiacheng He 5, 6, 7, 8, 9 , Michael Verano 3, 10, 11, 12, 13 , Ayoola T. Brimmo 1, 2, 3, 4, 14 , Ayoub Glia 1, 2, 3, 4, 14 , Mohammad A. Qasaimeh 1, 2, 3, 4, 14 , Pengyu Chen 5, 6, 7, 8, 9 , Jose O. Aleman 3, 10, 11, 12, 13 , Weiqiang Chen 1, 2, 3, 4, 15
Lab on a Chip ( IF 6.1 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1039/c8lc00605a Jingyi Zhu 1, 2, 3, 4 , Jiacheng He 5, 6, 7, 8, 9 , Michael Verano 3, 10, 11, 12, 13 , Ayoola T. Brimmo 1, 2, 3, 4, 14 , Ayoub Glia 1, 2, 3, 4, 14 , Mohammad A. Qasaimeh 1, 2, 3, 4, 14 , Pengyu Chen 5, 6, 7, 8, 9 , Jose O. Aleman 3, 10, 11, 12, 13 , Weiqiang Chen 1, 2, 3, 4, 15
Affiliation
|
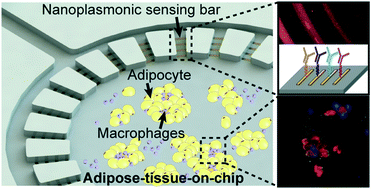
Although many advanced biosensing techniques have been proposed for cytokine profiling, there are no clinically available methods that integrate high-resolution immune cell monitoring and in situ multiplexed cytokine detection together in a biomimetic tissue microenvironment. The primary challenge arises due to the lack of suitable label-free sensing techniques and difficulty for sensor integration. In this work, we demonstrated a novel integration of a localized-surface plasmon resonance (LSPR)-based biosensor with a biomimetic microfluidic ‘adipose-tissue-on-chip’ platform for an in situ label-free, high-throughput and multiplexed cytokine secretion analysis of obese adipose tissue. Using our established adipose-tissue-on-chip platform, we were able to monitor the adipose tissue initiation, differentiation, and maturation and simulate the hallmark formation of crown-like structures (CLSs) during pro-inflammatory stimulation. With integrated antibody-conjugated LSPR barcode sensor arrays, our platform enables simultaneous multiplexed measurements of pro-inflammatory (IL-6 and TNF-α) and anti-inflammatory (IL-10 and IL-4) cytokines secreted by the adipocytes and macrophages. As a result, our adipose-tissue-on-chip platform is capable of identifying stage-specific cytokine secretion profiles from a complex milieu during obesity progression, highlighting its potential as a high-throughput preclinical readout for personalized obesity treatment strategies.
中文翻译:

集成的脂肪组织芯片纳米等离子体生物传感平台,用于研究与肥胖相关的炎症†
尽管已提出了许多先进的生物传感技术来进行细胞因子分析,但尚无临床可用的方法在仿生组织微环境中将高分辨率免疫细胞监测和原位多路细胞因子检测整合在一起。由于缺乏合适的无标签传感技术和传感器集成困难而引起了主要挑战。在这项工作中,我们展示了一种基于局部表面等离子体共振(LSPR)的生物传感器与仿生微流体“脂肪组织芯片”平台的新型集成,该平台可用于原位肥胖脂肪组织的无标记,高通量和多重细胞因子分泌分析。使用我们已建立的芯片上脂肪组织平台,我们能够监测促炎刺激过程中脂肪组织的起始,分化和成熟,并模拟冠状结构(CLS)的标志性形成。借助集成抗体结合的LSPR条码传感器阵列,我们的平台能够同时对脂肪细胞和巨噬细胞分泌的促炎性细胞因子(IL-6和TNF-α)和抗炎性细胞因子(IL-10和IL-4)进行多重测量。结果,我们的脂肪组织芯片平台能够在肥胖症发展过程中从复杂的环境中识别特定阶段的细胞因子分泌谱,
更新日期:2018-10-10
中文翻译:

集成的脂肪组织芯片纳米等离子体生物传感平台,用于研究与肥胖相关的炎症†
尽管已提出了许多先进的生物传感技术来进行细胞因子分析,但尚无临床可用的方法在仿生组织微环境中将高分辨率免疫细胞监测和原位多路细胞因子检测整合在一起。由于缺乏合适的无标签传感技术和传感器集成困难而引起了主要挑战。在这项工作中,我们展示了一种基于局部表面等离子体共振(LSPR)的生物传感器与仿生微流体“脂肪组织芯片”平台的新型集成,该平台可用于原位肥胖脂肪组织的无标记,高通量和多重细胞因子分泌分析。使用我们已建立的芯片上脂肪组织平台,我们能够监测促炎刺激过程中脂肪组织的起始,分化和成熟,并模拟冠状结构(CLS)的标志性形成。借助集成抗体结合的LSPR条码传感器阵列,我们的平台能够同时对脂肪细胞和巨噬细胞分泌的促炎性细胞因子(IL-6和TNF-α)和抗炎性细胞因子(IL-10和IL-4)进行多重测量。结果,我们的脂肪组织芯片平台能够在肥胖症发展过程中从复杂的环境中识别特定阶段的细胞因子分泌谱,











































 京公网安备 11010802027423号
京公网安备 11010802027423号