当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Luminescent ruffled iridium(iii) porphyrin complexes containing N-heterocyclic carbene ligands: structures, spectroscopies and potent antitumor activities under dark and light irradiation conditions†
Chemical Science ( IF 7.6 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1039/c8sc02920b Tsz-Lung Lam 1, 2, 3, 4, 5 , Ka-Chung Tong 1, 2, 3, 4, 5 , Chen Yang 1, 2, 3, 4, 5 , Wai-Lun Kwong 1, 2, 3, 4, 5 , Xiangguo Guan 1, 2, 3, 4, 5 , Ming-De Li 1, 2, 3, 4, 5 , Vanessa Kar-Yan Lo 1, 2, 3, 4, 5 , Sharon Lai-Fung Chan 5, 6, 7, 8 , David Lee Phillips 1, 2, 3, 4, 5 , Chun-Nam Lok 1, 2, 3, 4, 5 , Chi-Ming Che 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2018-10-10 00:00:00 , DOI: 10.1039/c8sc02920b Tsz-Lung Lam 1, 2, 3, 4, 5 , Ka-Chung Tong 1, 2, 3, 4, 5 , Chen Yang 1, 2, 3, 4, 5 , Wai-Lun Kwong 1, 2, 3, 4, 5 , Xiangguo Guan 1, 2, 3, 4, 5 , Ming-De Li 1, 2, 3, 4, 5 , Vanessa Kar-Yan Lo 1, 2, 3, 4, 5 , Sharon Lai-Fung Chan 5, 6, 7, 8 , David Lee Phillips 1, 2, 3, 4, 5 , Chun-Nam Lok 1, 2, 3, 4, 5 , Chi-Ming Che 1, 2, 3, 4, 5
Affiliation
|
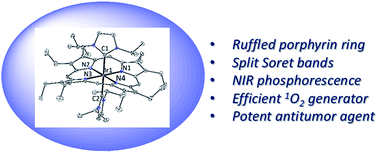
A panel of iridium(III) porphyrin complexes containing axial N-heterocyclic carbene (NHC) ligand(s) were synthesized and characterized. X-ray crystal structures of the bis-NHC complexes [IrIII(ttp)(IMe)2]+ (2a), [IrIII(oep)(BIMe)2]+ (2d), [IrIII(oep)(IiPr)2]+ (2e) and [IrIII(F20tpp)(IMe)2]+ (2f) display ruffled porphyrin rings with mesocarbon displacements of 0.483–0.594 Å and long Ir–CNHC bonds of 2.100–2.152 Å. Variable-temperature 1H NMR analysis of 2a reveals that the macrocycle porphyrin ring inversion takes place in solution with an activation barrier of 40 ± 1 kJ mol−1. The UV-vis absorption spectra of IrIII(por)–NHC complexes display split Soret bands. TD-DFT calculations and resonance Raman experiments show that the higher-energy Soret band is derived from the 1MLCT dπ(Ir) → π*(por) transition. The near-infrared phosphorescence of IrIII(por)–NHC complexes from the porphyrin-based 3(π, π*) state features broad emission bands at 701–754 nm with low emission quantum yields and short lifetimes (Φem < 0.01; τ < 4 μs). [IrIII(por)(IMe)2]+ complexes (por = ttp and oep) are efficient photosensitizers for 1O2 generation (Φso = 0.64 and 0.88) and are catalytically active in the light-induced aerobic oxidation of secondary amines and arylboronic acid. The bis-NHC complexes exhibit potent dark cytotoxicity towards a panel of cancer cells with IC50 values at submicromolar levels. The cytotoxicity of these complexes could be further enhanced upon light irradiation with IC50 values as low as nanomolar levels in association with the light-induced generation of reactive oxygen species (ROS). Bioimaging of [IrIII(oep)(IMe)2]+ (2c) treated cells indicates that this Ir complex mainly targets the endoplasmic reticulum. [IrIII(oep)(IMe)2]+ catalyzes the photoinduced generation of singlet oxygen and triggers protein oxidation, cell cycle arrest, apoptosis and the inhibition of angiogenesis. It also causes pronounced photoinduced inhibition of tumor growth in a mouse model of human cancer.
中文翻译:

含N-杂环卡宾配体的 发光皱纹铱(iii)卟啉配合物:在暗光条件下的结构,光谱学和有效的抗肿瘤活性†
合成并表征了一组包含轴向N-杂环卡宾(NHC)配体的铱(III)卟啉配合物。bis-NHC配合物[Ir III(ttp)(IMe)2 ] +(2a),[Ir III(oep)(BIMe)2 ] +(2d),[Ir III(oep)( I i Pr)2 ] +(2e)和[Ir III(F 20 tpp)(IMe)2 ] +(2f)显示了皱纹的卟啉环,中间碳的位移为0.483-0.594Å,长的Ir-C NHC键为2.100-2.152Å。2a的变温1 H NMR分析表明,大环卟啉环发生在具有40±1 kJ mol -1的激活势垒的溶液中。Ir III(por)–NHC配合物的紫外-可见吸收光谱显示出分裂的Soret带。TD-DFT计算和共振拉曼实验表明,较高能量的Soret带是从1 MLCTdπ(Ir)→π*(por)跃迁得出的。卟啉基3的Ir III(por)–NHC复合物的近红外磷光(π,π*)的状态在具有低发射量子产率和较短的寿命701-754纳米设有宽的发射频带(Φ EM <0.01; τ <4微秒)。物[Ir III(POR)(IME)2 ] +络合物(POR = TTP和OEP)是有效的光敏剂1 Ò 2代(Φ如此= 0.64和0.88),并在仲胺的光诱导的有氧氧化催化活性的和芳基硼酸。bis-NHC配合物对IC 50的癌细胞具有强大的黑暗细胞毒性作用值在亚微摩尔水平。这些复合物的细胞毒性可在光辐照下以低至纳摩尔水平的IC 50值与光诱导产生的活性氧(ROS)结合来进一步增强。[Ir III(oep)(IMe)2 ] +(2c)处理的细胞的生物成像表明,该Ir复合物主要靶向内质网。[Ir III(oep)(IMe)2 ] +催化单线态氧的光诱导产生并触发蛋白质氧化,细胞周期停滞,细胞凋亡和血管生成抑制。它还在人类癌症的小鼠模型中引起明显的光诱导的肿瘤生长抑制。
更新日期:2018-10-10
中文翻译:

含N-杂环卡宾配体的 发光皱纹铱(iii)卟啉配合物:在暗光条件下的结构,光谱学和有效的抗肿瘤活性†
合成并表征了一组包含轴向N-杂环卡宾(NHC)配体的铱(III)卟啉配合物。bis-NHC配合物[Ir III(ttp)(IMe)2 ] +(2a),[Ir III(oep)(BIMe)2 ] +(2d),[Ir III(oep)( I i Pr)2 ] +(2e)和[Ir III(F 20 tpp)(IMe)2 ] +(2f)显示了皱纹的卟啉环,中间碳的位移为0.483-0.594Å,长的Ir-C NHC键为2.100-2.152Å。2a的变温1 H NMR分析表明,大环卟啉环发生在具有40±1 kJ mol -1的激活势垒的溶液中。Ir III(por)–NHC配合物的紫外-可见吸收光谱显示出分裂的Soret带。TD-DFT计算和共振拉曼实验表明,较高能量的Soret带是从1 MLCTdπ(Ir)→π*(por)跃迁得出的。卟啉基3的Ir III(por)–NHC复合物的近红外磷光(π,π*)的状态在具有低发射量子产率和较短的寿命701-754纳米设有宽的发射频带(Φ EM <0.01; τ <4微秒)。物[Ir III(POR)(IME)2 ] +络合物(POR = TTP和OEP)是有效的光敏剂1 Ò 2代(Φ如此= 0.64和0.88),并在仲胺的光诱导的有氧氧化催化活性的和芳基硼酸。bis-NHC配合物对IC 50的癌细胞具有强大的黑暗细胞毒性作用值在亚微摩尔水平。这些复合物的细胞毒性可在光辐照下以低至纳摩尔水平的IC 50值与光诱导产生的活性氧(ROS)结合来进一步增强。[Ir III(oep)(IMe)2 ] +(2c)处理的细胞的生物成像表明,该Ir复合物主要靶向内质网。[Ir III(oep)(IMe)2 ] +催化单线态氧的光诱导产生并触发蛋白质氧化,细胞周期停滞,细胞凋亡和血管生成抑制。它还在人类癌症的小鼠模型中引起明显的光诱导的肿瘤生长抑制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号