当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Dispersive Gold Nanoparticles on Carbon Black for Oxygen and Carbon Dioxide Reduction
Electroanalysis ( IF 2.7 ) Pub Date : 2018-10-10 , DOI: 10.1002/elan.201800555 Yeomin Kim 1 , Ara Jo 1 , Yejin Ha 1 , Yongjin Lee 2 , Dongil Lee 2 , Youngmi Lee 1 , Chongmok Lee 1
Electroanalysis ( IF 2.7 ) Pub Date : 2018-10-10 , DOI: 10.1002/elan.201800555 Yeomin Kim 1 , Ara Jo 1 , Yejin Ha 1 , Yongjin Lee 2 , Dongil Lee 2 , Youngmi Lee 1 , Chongmok Lee 1
Affiliation
|
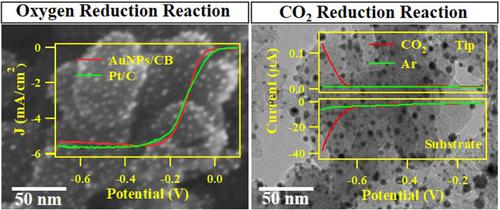
Highly dispersive Au nanoparticles on carbon black (Au NPs/CB) were synthesized in situ with co‐present two different reducing agents of NaBH4 at various concentrations and citrate at a constant concentration of 3 mM. The average diameters of Au NPs on carbon support were in the range from 5.8 (±2.4) to 2.0 (±0.4) nm, with 50 particles quantified. Electrocatalytic activities of as‐prepared Au NPs/CB were explored for oxygen reduction reaction (ORR) in basic solution with rotating disk electrode (RDE) and rotating ring‐disk electrode (RRDE) voltammetry. In results, the Au NPs/CB synthesized with 0.2 mM NaBH4 (2.0 nm of Au NPs diameter) represented the highest ORR catalytic activity with electron transfer number of 3.9 and mass activity of 0.25 mA cm−2 μg−1as well as a perfect resistance to methanol contamination. Especially, the half‐wave potential of ORR curve which related to the kinetics of oxygen reduction was more positive compared with previously reported Au‐based ORR catalysts. In addition, the Au NPs/CB prepared with 0.2 mM NaBH4 was also examined as a CO2 reduction catalyst in KHCO3 with KCl solution with scanning electrochemical microscopy (SECM). CO2 was reduced to CO selectively without hydrogen evolution at Au NPs/CB substrate electrode, which was directly monitored with an electrochemical CO microsensor as a tip electrode in SECM. In addition, we have identified the products of CO2 reduction through gas chromatography (GC)‐mass spectrometry (MS), flame ionization detector (FID), and thermal conductivity detector (TCD).
中文翻译:

炭黑上高度分散的金纳米颗粒,用于减少氧气和二氧化碳
在炭黑上高度分散的金纳米颗粒(Au NPs / CB)与NaBH 4的两种不同还原剂以不同浓度原位合成,并以3 mM的恒定浓度柠檬酸盐共存。碳载体上的金纳米颗粒的平均直径在5.8(±2.4)到2.0(±0.4)nm的范围内,其中有50个颗粒被量化。利用旋转盘电极(RDE)和旋转环盘电极(RRDE)伏安法研究了制备的Au NPs / CB在碱性溶液中的氧还原反应(ORR)的电催化活性。结果表明,用0.2 mM NaBH 4(直径为2.0 nm的Au NPs合成)合成的Au NPs / CB表现出最高的ORR催化活性,电子转移数为3.9,质量活性为0.25 mA cm-2 微克-1以及甲醇污染的理想电阻。尤其是,与先前报道的金基ORR催化剂相比,与氧还原动力学相关的ORR曲线的半波电势更正。另外,还用扫描电化学显微镜(SECM)检查了用0.2mM NaBH 4制备的Au NPs / CB作为KHCO 3和KCl溶液中的CO 2还原催化剂。在Au NPs / CB衬底电极上,CO 2选择性地还原为CO而没有氢放出,这可以通过电化学CO微传感器作为SECM中的尖端电极直接进行监测。此外,我们还确定了CO 2的产物 通过气相色谱(GC)-质谱(MS),火焰离子化检测器(FID)和热导检测器(TCD)进行还原。
更新日期:2018-10-10
中文翻译:

炭黑上高度分散的金纳米颗粒,用于减少氧气和二氧化碳
在炭黑上高度分散的金纳米颗粒(Au NPs / CB)与NaBH 4的两种不同还原剂以不同浓度原位合成,并以3 mM的恒定浓度柠檬酸盐共存。碳载体上的金纳米颗粒的平均直径在5.8(±2.4)到2.0(±0.4)nm的范围内,其中有50个颗粒被量化。利用旋转盘电极(RDE)和旋转环盘电极(RRDE)伏安法研究了制备的Au NPs / CB在碱性溶液中的氧还原反应(ORR)的电催化活性。结果表明,用0.2 mM NaBH 4(直径为2.0 nm的Au NPs合成)合成的Au NPs / CB表现出最高的ORR催化活性,电子转移数为3.9,质量活性为0.25 mA cm-2 微克-1以及甲醇污染的理想电阻。尤其是,与先前报道的金基ORR催化剂相比,与氧还原动力学相关的ORR曲线的半波电势更正。另外,还用扫描电化学显微镜(SECM)检查了用0.2mM NaBH 4制备的Au NPs / CB作为KHCO 3和KCl溶液中的CO 2还原催化剂。在Au NPs / CB衬底电极上,CO 2选择性地还原为CO而没有氢放出,这可以通过电化学CO微传感器作为SECM中的尖端电极直接进行监测。此外,我们还确定了CO 2的产物 通过气相色谱(GC)-质谱(MS),火焰离子化检测器(FID)和热导检测器(TCD)进行还原。











































 京公网安备 11010802027423号
京公网安备 11010802027423号