当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Pharmacological Characterization of Carbazole Based Dopamine Agonists as Potential Symptomatic and Neuroprotective Therapeutic Agents for Parkinson's Disease.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-10-24 , DOI: 10.1021/acschemneuro.8b00291 Asma Elmabruk 1 , Banibrata Das 1 , Deepthi Yedlapudi 1 , Liping Xu 1 , Tamara Antonio 2 , Maarten E A Reith 2 , Aloke K Dutta 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2018-10-24 , DOI: 10.1021/acschemneuro.8b00291 Asma Elmabruk 1 , Banibrata Das 1 , Deepthi Yedlapudi 1 , Liping Xu 1 , Tamara Antonio 2 , Maarten E A Reith 2 , Aloke K Dutta 1
Affiliation
|
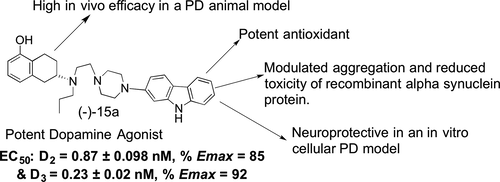
We have developed a series of carbazole-derived compounds based on our hybrid D2/D3 agonist template to design multifunctional compounds for the symptomatic and disease-modifying treatment of Parkinson's disease (PD). The lead molecules (-)-11b (D-636), (-)-15a (D-653), and (-)-15c (D-656) exhibited high affinity for both D2 and D3 receptors and in GTPγS functional assay, the compounds showed potent agonist activity at both D2 and D3 receptors (EC50 (GTPγS); D2 = 48.7 nM, D3 = 0.96 nM for 11b, D2 = 0.87 nM, D3 = 0.23 nM for 15a and D2 = 2.29 nM, D3 = 0.22 nM for 15c). In an animal model of PD, the test compounds exhibited potent in vivo activity in reversing hypolocomotion in reserpinized rats with a long duration of action compared to the reference drug ropinirole. In a cellular antioxidant assay, compounds (-)-11b, (-)-15a, and (-)-15c exhibited potent activity in reducing oxidative stress induced by neurotoxin 6-hydroxydopamine (6-OHDA). Also, in a cell-based PD neuroprotection model, these lead compounds significantly increased cell survival from toxicity of 6-OHDA, thereby producing a neuroprotective effect. Additionally, compounds (-)-11b and (-)-15a inhibited aggregation and reduced toxicity of recombinant alpha synuclein protein in a cell based in vitro assay. These observations suggest that the lead carbazole-based dopamine agonists may be promising multifunctional molecules for a viable symptomatic and disease-modifying therapy of PD and should be further investigated.
中文翻译:

基于咔唑的多巴胺激动剂作为帕金森病潜在症状和神经保护治疗剂的设计、合成和药理学表征。
我们开发了一系列基于我们的混合 D2/D3 激动剂模板的咔唑衍生化合物,以设计多功能化合物,用于帕金森病 (PD) 的对症和疾病缓解治疗。先导分子 (-)-11b (D-636)、(-)-15a (D-653) 和 (-)-15c (D-656) 对 D2 和 D3 受体以及 GTPγS 功能测定均表现出高亲和力,这些化合物对 D2 和 D3 受体均显示出有效的激动剂活性(EC50 (GTPγS);对于 11b,D2 = 48.7 nM,D3 = 0.96 nM,对于 15a,D2 = 0.87 nM,D3 = 0.23 nM,D2 = 2.23 = 2.29 nM,D 15c 为 0.22 nM)。在 PD 动物模型中,与参考药物罗匹尼罗相比,受试化合物在逆转利血平大鼠的运动不足方面表现出强大的体内活性,作用持续时间长。在细胞抗氧化试验中,化合物 (-)-11b、(-)-15a、和 (-)-15c 在减少由神经毒素 6-羟基多巴胺 (6-OHDA) 诱导的氧化应激方面表现出有效的活性。此外,在基于细胞的 PD 神经保护模型中,这些先导化合物显着增加了 6-OHDA 毒性的细胞存活率,从而产生了神经保护作用。此外,化合物 (-)-11b 和 (-)-15a 在基于细胞的体外测定中抑制了重组 α 突触核蛋白的聚集并降低了毒性。这些观察结果表明,基于咔唑的多巴胺激动剂可能是一种很有前途的多功能分子,可用于 PD 的可行的对症和疾病缓解治疗,应进一步研究。这些先导化合物显着增加了 6-OHDA 毒性的细胞存活率,从而产生了神经保护作用。此外,化合物 (-)-11b 和 (-)-15a 在基于细胞的体外测定中抑制了重组 α 突触核蛋白的聚集并降低了毒性。这些观察结果表明,基于咔唑的多巴胺激动剂可能是一种很有前途的多功能分子,可用于 PD 的可行的对症和疾病缓解治疗,应进一步研究。这些先导化合物显着增加了 6-OHDA 毒性的细胞存活率,从而产生了神经保护作用。此外,化合物 (-)-11b 和 (-)-15a 在基于细胞的体外测定中抑制了重组 α 突触核蛋白的聚集并降低了毒性。这些观察结果表明,基于咔唑的多巴胺激动剂可能是一种很有前途的多功能分子,可用于 PD 的可行的对症和疾病缓解治疗,应进一步研究。
更新日期:2018-10-09
中文翻译:

基于咔唑的多巴胺激动剂作为帕金森病潜在症状和神经保护治疗剂的设计、合成和药理学表征。
我们开发了一系列基于我们的混合 D2/D3 激动剂模板的咔唑衍生化合物,以设计多功能化合物,用于帕金森病 (PD) 的对症和疾病缓解治疗。先导分子 (-)-11b (D-636)、(-)-15a (D-653) 和 (-)-15c (D-656) 对 D2 和 D3 受体以及 GTPγS 功能测定均表现出高亲和力,这些化合物对 D2 和 D3 受体均显示出有效的激动剂活性(EC50 (GTPγS);对于 11b,D2 = 48.7 nM,D3 = 0.96 nM,对于 15a,D2 = 0.87 nM,D3 = 0.23 nM,D2 = 2.23 = 2.29 nM,D 15c 为 0.22 nM)。在 PD 动物模型中,与参考药物罗匹尼罗相比,受试化合物在逆转利血平大鼠的运动不足方面表现出强大的体内活性,作用持续时间长。在细胞抗氧化试验中,化合物 (-)-11b、(-)-15a、和 (-)-15c 在减少由神经毒素 6-羟基多巴胺 (6-OHDA) 诱导的氧化应激方面表现出有效的活性。此外,在基于细胞的 PD 神经保护模型中,这些先导化合物显着增加了 6-OHDA 毒性的细胞存活率,从而产生了神经保护作用。此外,化合物 (-)-11b 和 (-)-15a 在基于细胞的体外测定中抑制了重组 α 突触核蛋白的聚集并降低了毒性。这些观察结果表明,基于咔唑的多巴胺激动剂可能是一种很有前途的多功能分子,可用于 PD 的可行的对症和疾病缓解治疗,应进一步研究。这些先导化合物显着增加了 6-OHDA 毒性的细胞存活率,从而产生了神经保护作用。此外,化合物 (-)-11b 和 (-)-15a 在基于细胞的体外测定中抑制了重组 α 突触核蛋白的聚集并降低了毒性。这些观察结果表明,基于咔唑的多巴胺激动剂可能是一种很有前途的多功能分子,可用于 PD 的可行的对症和疾病缓解治疗,应进一步研究。这些先导化合物显着增加了 6-OHDA 毒性的细胞存活率,从而产生了神经保护作用。此外,化合物 (-)-11b 和 (-)-15a 在基于细胞的体外测定中抑制了重组 α 突触核蛋白的聚集并降低了毒性。这些观察结果表明,基于咔唑的多巴胺激动剂可能是一种很有前途的多功能分子,可用于 PD 的可行的对症和疾病缓解治疗,应进一步研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号