Tetrahedron Letters ( IF 1.5 ) Pub Date : 2018-10-10 , DOI: 10.1016/j.tetlet.2018.10.015 Tadashi Aoyama , Kenshiro Tashiro , Mamiko Hayakawa , Shigeru Shimada , Akihiko Ouchi
|
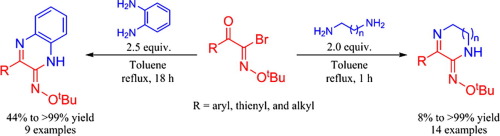
A simple and efficient method was developed for the synthesis of 5,6-dihydropyrazin-2(1H)-one O-(tert-butyl)oximes, quinoxalin-2(1H)-one O-(tert-butyl)oximes and its derivatives from N-tert-butoxy acyl imidoyl bromide and diamines. Twenty three novel compounds were readily synthesized using this procedure in excellent yields. The products possessed Z-stereochemistry with regard to the CN double bond. In these reactions, the amino group first attacked an imidoyl carbon of the N-tert-butoxy acyl imidoyl bromide in order to obtain the Z-intermediate; then another amino group attacked the carbonyl carbon of the intermediate. The rate of intramolecular cyclization of the intermediate was dependent on the position of the diamino groups.
中文翻译:

一种简单高效的合成5,6-二氢吡嗪-2(1 H)-一O-(叔丁基)肟,喹喔啉-2(1 H)-1一O-(叔丁基)肟的方法衍生品
建立了一种简单有效的合成5,6-二氢吡嗪-2(1 H)-一O-(叔丁基)肟,喹喔啉-2(1 H)-一O-(叔丁基)肟的方法。和其衍生物从ñ -叔丁氧基酰亚胺酰基溴和二胺。使用该方法很容易合成了23种新型化合物,收率很高。产物具有关于C N双键的Z-立体化学。在这些反应中,氨基第一攻击的亚氨碳ñ -叔丁氧基酰亚胺溴化以获得Z-中间体; 然后另一个氨基攻击中间体的羰基碳。中间体的分子内环化速率取决于二氨基的位置。











































 京公网安备 11010802027423号
京公网安备 11010802027423号