当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction-Induced Excitations and Their Effect on Surface Chemistry
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-09 00:00:00 , DOI: 10.1021/acscatal.8b03266 Matthew M. Montemore 1, 2 , Robert Hoyt 3 , Grigory Kolesov 2 , Efthimios Kaxiras 2, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-09 00:00:00 , DOI: 10.1021/acscatal.8b03266 Matthew M. Montemore 1, 2 , Robert Hoyt 3 , Grigory Kolesov 2 , Efthimios Kaxiras 2, 3
Affiliation
|
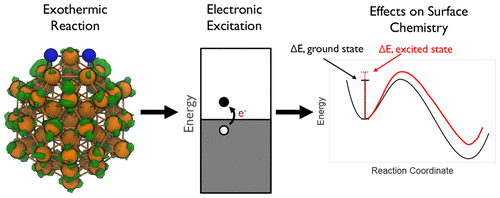
Despite intensive study of reactions on metals, it is unclear whether electronic excitations play an important role. Here, we show that nonadiabatic effects do indeed play a significant role in N2 and H2 dissociation on Ru nanoparticles. We employ nonadiabatic dynamical calculations based on real-time, time-dependent density functional theory to study energy dissipation during these exothermic reaction steps. We find that dissipation of the excess energy into excitation of electrons exceeds thermal dissipation into phonons. For isolated dissociation events, electronic friction can increase reaction barriers; furthermore, the excitations induced by a dissociation event can affect other reacting molecules. Our studies suggest that, for exothermic reactions, metal catalysts in reaction conditions may be constantly experiencing electronic excitations, and these excitations can significantly affect surface chemistry.
中文翻译:

反应诱导的兴奋及其对表面化学的影响
尽管对金属反应进行了深入研究,但尚不清楚电子激发是否起重要作用。在这里,我们显示非绝热效应确实在N 2和H 2中起着重要作用在Ru纳米颗粒上解离。我们采用基于实时,随时间变化的密度泛函理论的非绝热动力学计算来研究这些放热反应步骤中的能量耗散。我们发现,多余能量在电子激发中的耗散超过了热能在声子中的耗散。对于孤立的解离事件,电子摩擦会增加反应障碍。此外,解离事件引起的激发会影响其他反应分子。我们的研究表明,对于放热反应,反应条件下的金属催化剂可能会不断受到电子激发,这些激发会显着影响表面化学。
更新日期:2018-10-09
中文翻译:

反应诱导的兴奋及其对表面化学的影响
尽管对金属反应进行了深入研究,但尚不清楚电子激发是否起重要作用。在这里,我们显示非绝热效应确实在N 2和H 2中起着重要作用在Ru纳米颗粒上解离。我们采用基于实时,随时间变化的密度泛函理论的非绝热动力学计算来研究这些放热反应步骤中的能量耗散。我们发现,多余能量在电子激发中的耗散超过了热能在声子中的耗散。对于孤立的解离事件,电子摩擦会增加反应障碍。此外,解离事件引起的激发会影响其他反应分子。我们的研究表明,对于放热反应,反应条件下的金属催化剂可能会不断受到电子激发,这些激发会显着影响表面化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号