当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Study of Mycobacterium smegmatis Acyl Transferase Reaction Mechanism and Specificity
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-09 00:00:00 , DOI: 10.1021/acscatal.8b03360 Masoud Kazemi 1 , Xiang Sheng 1 , Wolfgang Kroutil 2 , Fahmi Himo 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2018-10-09 00:00:00 , DOI: 10.1021/acscatal.8b03360 Masoud Kazemi 1 , Xiang Sheng 1 , Wolfgang Kroutil 2 , Fahmi Himo 1
Affiliation
|
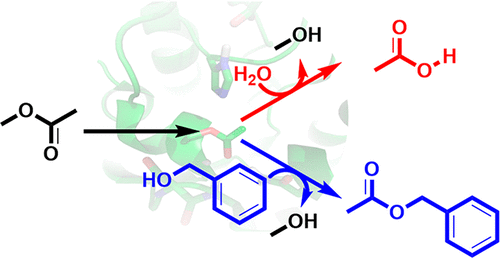
The acyl transferase from Mycobacterium smegmatis (MsAcT) catalyzes the acyl transfer between a range of primary and secondary alcohols, whereby its outstanding ability is to perform this reaction in aqueous solution. Therefore, MsAcT opens different options for acylation reactions enabling alternatives for many conventionally hydrolytic enzymes used in biocatalysis. Nevertheless, hydrolysis is still a major side reaction of this enzyme. To provide a detailed understanding of the competition between hydrolysis and transesterification reactions, a combination of density functional theory and free energy perturbation methods have been employed. The relative binding free energies and the energy profiles of the chemical steps involved in the reaction were calculated for a number of substrates. The calculations show that the enzyme active site exhibits a higher affinity for substrates with an aromatic ring. The rate-determining step corresponds to the collapse of a negatively charged tetrahedral intermediate in the substrate acylation half-reaction. The intrinsic barriers of the transesterification and hydrolysis half-reactions are calculated to be of similar heights, suggesting that the determining factor in the MsAcT specificity is the higher binding affinity of the active site for the alcohol substrates relative to water. Finally, the influence of the acyl donor on the MsAcT-catalyzed reaction is also investigated by considering different esters in the calculations.
中文翻译:

耻垢分枝杆菌酰基转移酶反应机理和特异性的计算研究
耻垢分枝杆菌的酰基转移酶(MsAcT)催化一定范围的伯醇和仲醇之间的酰基转移,因此其杰出的能力是在水溶液中进行该反应。因此,MsAcT为酰化反应打开了不同的选择,从而为生物催化中使用的许多常规水解酶提供了替代方案。然而,水解仍然是该酶的主要副反应。为了提供对水解和酯交换反应之间竞争的详细了解,已采用了密度泛函理论和自由能扰动方法的组合。对于许多底物,计算了反应中涉及的化学步骤的相对结合自由能和能谱。计算表明,酶活性位点对具有芳香环的底物表现出更高的亲和力。速率确定步骤对应于底物酰化半反应中带负电的四面体中间体的崩溃。计算出酯交换作用和水解半反应的固有障碍具有相似的高度,这表明MsAcT特异性的决定因素是活性位点对醇底物相对于水的更高结合亲和力。最后,通过在计算中考虑不同的酯,还研究了酰基供体对MsAcT催化的反应的影响。计算出酯交换作用和水解半反应的固有障碍具有相似的高度,这表明MsAcT特异性的决定因素是活性位点对醇底物相对于水的更高结合亲和力。最后,通过在计算中考虑不同的酯,还研究了酰基供体对MsAcT催化的反应的影响。计算出酯交换作用和水解半反应的固有障碍具有相似的高度,这表明MsAcT特异性的决定因素是活性位点对醇底物相对于水的更高结合亲和力。最后,通过在计算中考虑不同的酯,还研究了酰基供体对MsAcT催化的反应的影响。
更新日期:2018-10-09
中文翻译:

耻垢分枝杆菌酰基转移酶反应机理和特异性的计算研究
耻垢分枝杆菌的酰基转移酶(MsAcT)催化一定范围的伯醇和仲醇之间的酰基转移,因此其杰出的能力是在水溶液中进行该反应。因此,MsAcT为酰化反应打开了不同的选择,从而为生物催化中使用的许多常规水解酶提供了替代方案。然而,水解仍然是该酶的主要副反应。为了提供对水解和酯交换反应之间竞争的详细了解,已采用了密度泛函理论和自由能扰动方法的组合。对于许多底物,计算了反应中涉及的化学步骤的相对结合自由能和能谱。计算表明,酶活性位点对具有芳香环的底物表现出更高的亲和力。速率确定步骤对应于底物酰化半反应中带负电的四面体中间体的崩溃。计算出酯交换作用和水解半反应的固有障碍具有相似的高度,这表明MsAcT特异性的决定因素是活性位点对醇底物相对于水的更高结合亲和力。最后,通过在计算中考虑不同的酯,还研究了酰基供体对MsAcT催化的反应的影响。计算出酯交换作用和水解半反应的固有障碍具有相似的高度,这表明MsAcT特异性的决定因素是活性位点对醇底物相对于水的更高结合亲和力。最后,通过在计算中考虑不同的酯,还研究了酰基供体对MsAcT催化的反应的影响。计算出酯交换作用和水解半反应的固有障碍具有相似的高度,这表明MsAcT特异性的决定因素是活性位点对醇底物相对于水的更高结合亲和力。最后,通过在计算中考虑不同的酯,还研究了酰基供体对MsAcT催化的反应的影响。










































 京公网安备 11010802027423号
京公网安备 11010802027423号