当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A cascade synthesis of S-allyl benzoylcarbamothioates via Mumm-type rearrangement
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-10-09 , DOI: 10.1039/c8ob02293c Anjali Dahiya 1, 2, 3 , Wajid Ali 1, 2, 3 , Tipu Alam 1, 2, 3 , Bhisma K. Patel 1, 2, 3
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-10-09 , DOI: 10.1039/c8ob02293c Anjali Dahiya 1, 2, 3 , Wajid Ali 1, 2, 3 , Tipu Alam 1, 2, 3 , Bhisma K. Patel 1, 2, 3
Affiliation
|
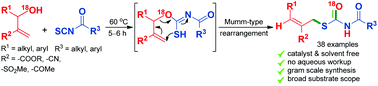
A catalyst and solvent free synthesis of S-allyl benzoylcarbamothioates has been achieved from the in situ generated benzoylcarbonimidothioates obtained by reacting MBH alcohols with aroyl isothiocyanates. An intramolecular thia-Michael addition of the in situ generated adduct triggers a Mumm-type rearrangement leading to a stereoselective synthesis of highly functionalised S-allyl benzoylcarbamothioates.
中文翻译:

通过Mumm型重排进行级联合成S-烯丙基苯甲酰氨基硫代酸酯
由通过使MBH醇与芳基异硫氰酸酯反应获得的原位生成的苯甲酰基碳亚氨基硫代酸酯已经实现了S-烯丙基苯甲酰基氨基甲硫代酸酯的无催化剂和无溶剂的合成。原位产生的加合物的分子内硫代-迈克尔加成引发Mumm型重排,导致高度官能化的S-烯丙基苯甲酰基氨基甲酸酯的立体选择性合成。
更新日期:2018-11-02
中文翻译:

通过Mumm型重排进行级联合成S-烯丙基苯甲酰氨基硫代酸酯
由通过使MBH醇与芳基异硫氰酸酯反应获得的原位生成的苯甲酰基碳亚氨基硫代酸酯已经实现了S-烯丙基苯甲酰基氨基甲硫代酸酯的无催化剂和无溶剂的合成。原位产生的加合物的分子内硫代-迈克尔加成引发Mumm型重排,导致高度官能化的S-烯丙基苯甲酰基氨基甲酸酯的立体选择性合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号