当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Four-component acyloxy-trifluoromethylation of arylalkenes mediated by a photoredox catalyst
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-10-09 , DOI: 10.1039/c8ob02239a Xiaocong Zhou 1, 2, 3, 4, 5 , Guijie Li 1, 2, 3, 4 , Zongzhou Shao 1, 2, 3, 4 , Kun Fang 1, 2, 3, 4 , Hongjun Gao 3, 4, 5, 6 , Yuanqiang Li 3, 4, 5, 6 , Yuanbin She 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-10-09 , DOI: 10.1039/c8ob02239a Xiaocong Zhou 1, 2, 3, 4, 5 , Guijie Li 1, 2, 3, 4 , Zongzhou Shao 1, 2, 3, 4 , Kun Fang 1, 2, 3, 4 , Hongjun Gao 3, 4, 5, 6 , Yuanqiang Li 3, 4, 5, 6 , Yuanbin She 1, 2, 3, 4
Affiliation
|
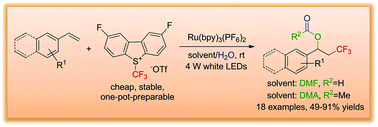
A four-component intermolecular trifluoromethylation–acyloxylation of arylalkenes induced by visible light has been developed in the presence of the photoredox catalyst Ru(bpy)3(PF6)2 under mild reaction conditions. A new Umemoto's reagent was used as a trifluoromethyl radical source, and this redox neutral reaction demonstrated good functional group tolerance for aryl alkenes with high yields up to 91%. The detailed reaction process was investigated based on control, deuterium and O18-labeling experiments to support that N,N-dimethylformamide (DMF)/H2O acted as an acyloxyl source.
中文翻译:

光氧化还原催化剂介导的芳基烯烃的四组分酰氧基-三氟甲基化
在光氧化还原催化剂Ru(bpy)3(PF 6)2的存在下,在温和的反应条件下,开发了由可见光诱导的芳烃的四组分分子间三氟甲基化-酰氧基化反应。一种新的梅本试剂用作三氟甲基自由基的来源,该氧化还原中性反应显示出对芳基烯烃具有良好的官能团耐受性,收率高达91%。基于对照,氘和O 18标记实验研究了详细的反应过程,以支持N,N-二甲基甲酰胺(DMF)/ H 2 O充当酰氧基源。
更新日期:2018-12-20
中文翻译:

光氧化还原催化剂介导的芳基烯烃的四组分酰氧基-三氟甲基化
在光氧化还原催化剂Ru(bpy)3(PF 6)2的存在下,在温和的反应条件下,开发了由可见光诱导的芳烃的四组分分子间三氟甲基化-酰氧基化反应。一种新的梅本试剂用作三氟甲基自由基的来源,该氧化还原中性反应显示出对芳基烯烃具有良好的官能团耐受性,收率高达91%。基于对照,氘和O 18标记实验研究了详细的反应过程,以支持N,N-二甲基甲酰胺(DMF)/ H 2 O充当酰氧基源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号