当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The peculiar effect of water on ionic liquids and deep eutectic solvents
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-10-09 00:00:00 , DOI: 10.1039/c8cs00325d Chunyan Ma 1, 2, 3, 4, 5 , Aatto Laaksonen 6, 7, 8, 9, 10 , Chang Liu 6, 7, 8, 9 , Xiaohua Lu 6, 7, 8, 9 , Xiaoyan Ji 1, 2, 3, 4, 5
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2018-10-09 00:00:00 , DOI: 10.1039/c8cs00325d Chunyan Ma 1, 2, 3, 4, 5 , Aatto Laaksonen 6, 7, 8, 9, 10 , Chang Liu 6, 7, 8, 9 , Xiaohua Lu 6, 7, 8, 9 , Xiaoyan Ji 1, 2, 3, 4, 5
Affiliation
|
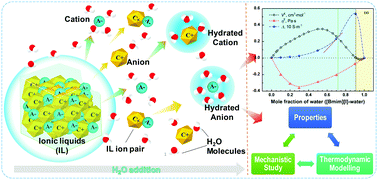
Ionic liquids (ILs) and deep eutectic solvents (DESs) have been suggested as eco-friendly alternatives to organic solvents. A trace amount of water is often unavoidable as impurity, and water is also added on purpose to reduce their problematically high viscosity and lower their high price. Understanding the distinct effects of water on the properties of ILs/DESs is highly important. In this review, we collect published experimental and theoretical results for IL/DES–H2O systems at varied water concentrations and analyze them. Results from mechanistic studies, thermodynamic modelling and advanced experiments are collected and critically discussed. Six commonly studied IL/DES–H2O systems were selected to map experimental observations onto microscopic results obtained in mechanistic studies. A great variety of distinct contours of the excess properties can be observed over the entire compositional range, indicating that the properties of IL/DES–H2O systems are highly unpredictable. Mechanistic studies clearly demonstrate that the added H2O rapidly changes the heterogeneous 3D structures of pure ILs/DESs, leading to very different properties and behaviour. There are similarities between aqueous electrolytes and IL/DES solutions but the bulky and asymmetric organic cations in ILs/DESs do not conform to the standard salt dissolution and hydration concepts. Thermodynamic modelling previously assumes ILs/DESs to be either a neutral ion-pair or completely dissociated ions, neglecting specific ion hydration effects. A new conceptual framework is suggested for thermodynamic modelling of IL/DES–H2O binary systems to enable new technologies for their practical applications.
中文翻译:

水对离子液体和深共熔溶剂的特殊作用
离子液体(ILs)和深共熔溶剂(DESs)已被建议作为有机溶剂的环保替代品。常常不可避免地会留下痕量的水作为杂质,并且故意添加水以降低其有问题的高粘度并降低其高价。了解水对ILs / DESs性质的不同影响非常重要。在这篇综述中,我们收集了不同水浓度下IL / DES–H 2 O系统的已发表实验和理论结果,并对它们进行了分析。机理研究,热力学建模和高级实验的结果被收集并进行了严格的讨论。六种常用的IL / DES–H 2选择O系统将实验观察结果映射到在机械研究中获得的微观结果上。在整个组成范围内,可以观察到多种多样的过剩特性轮廓,这表明IL / DES–H 2 O系统的特性是高度不可预测的。机理研究清楚地表明,添加的H 2O会迅速改变纯IL / DES的异构3D结构,从而导致性质和行为截然不同。水性电解质和IL / DES溶液之间存在相似之处,但是ILs / DES中庞大且不对称的有机阳离子不符合标准的盐溶解和水合概念。热力学建模以前假设IL / DES为中性离子对或完全解离的离子,而忽略了特定的离子水合效应。建议为IL / DES–H 2 O二元系统的热力学建模提供一个新的概念框架,以使新技术可以在其实际应用中使用。
更新日期:2018-10-09
中文翻译:

水对离子液体和深共熔溶剂的特殊作用
离子液体(ILs)和深共熔溶剂(DESs)已被建议作为有机溶剂的环保替代品。常常不可避免地会留下痕量的水作为杂质,并且故意添加水以降低其有问题的高粘度并降低其高价。了解水对ILs / DESs性质的不同影响非常重要。在这篇综述中,我们收集了不同水浓度下IL / DES–H 2 O系统的已发表实验和理论结果,并对它们进行了分析。机理研究,热力学建模和高级实验的结果被收集并进行了严格的讨论。六种常用的IL / DES–H 2选择O系统将实验观察结果映射到在机械研究中获得的微观结果上。在整个组成范围内,可以观察到多种多样的过剩特性轮廓,这表明IL / DES–H 2 O系统的特性是高度不可预测的。机理研究清楚地表明,添加的H 2O会迅速改变纯IL / DES的异构3D结构,从而导致性质和行为截然不同。水性电解质和IL / DES溶液之间存在相似之处,但是ILs / DES中庞大且不对称的有机阳离子不符合标准的盐溶解和水合概念。热力学建模以前假设IL / DES为中性离子对或完全解离的离子,而忽略了特定的离子水合效应。建议为IL / DES–H 2 O二元系统的热力学建模提供一个新的概念框架,以使新技术可以在其实际应用中使用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号